List Of The 118 Known Chemical Elements
The following sortable table shows the 118 known chemical elements.
- Atomic number, Element, and Symbol all serve independently as unique identifiers.
- Element names are those accepted by IUPAC.
- Symbol column background color indicates the periodic table block for each element: red = s-block, yellow = p-block, blue = d-block, green = f-block.
- Group and period refer to an element’s position in the periodic table. Group numbers here show the currently accepted numbering for older alternate numberings, see Group .
What Is A Mechanical Mixture
A mechanical mixture is one that can be separated through mechanical means, such as a magnet or a centrifuge. Mechanical mixtures are not chemically bonded.
While mechanical mixtures may be attracted to each other, there is no permanent gain or loss of electrons they do not form different molecules.
One example of a mechanical mixture is iron filings in flour. No matter how thoroughly they are mixed, the iron filings can always be separated from the flour using a magnet. Water with microscopic particles in it is a mechanical mixture, as these particles can be removed through filtration. Oil floating on water is a mechanical mixture because oil and water do not combine. Even blood can be separated into its components, as red cells are heavier than plasma. However, salt water is not a mechanical mixture because the ions in salt are bonded to the water molecules. The salt cannot be extracted without, for example, the application of heat to cause a chemical change.
Mechanical mixtures are often created in recycling. Magnets, filtration and other methods are used to separate each recyclable component, as they have different physical properties. Given the lack of chemical bonds, it is also possible to apply chemicals that only affect one component of the mixture.
What Is A Mixture In Science
In science, specifically in chemistry, a mixture is when you combine two or more substances and each of the substances retains its own chemical makeup. To be a mixture, the substances cannot form or break chemical bonds with each other.
In order to be considered a mixture, the substances must satisfy three general properties. The components in a mixture can easily be separated, they each keep their own chemical properties and the proportion of the components is variable.
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers
Homogeneous Vs Heterogeneous Mixture
In chemistry, we can have two types of mixtures: homogeneous mixtures and heterogeneous mixtures:
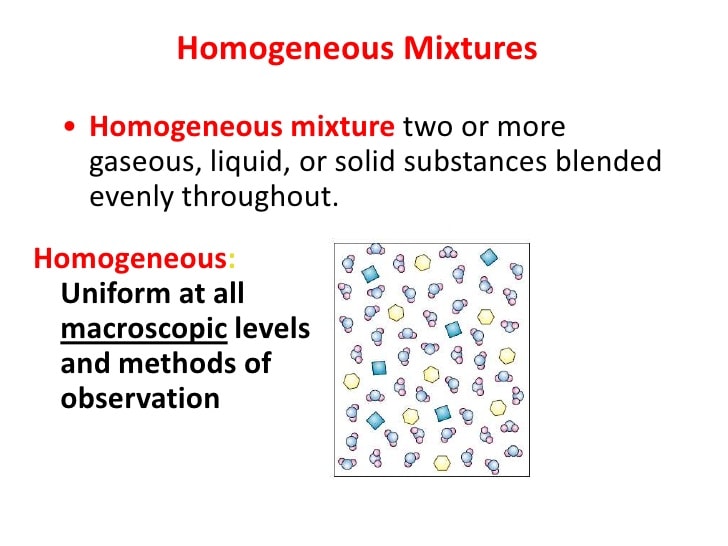
- Homogeneous mixture: Blended so thoroughly, it looks like one substance Uniform composition.
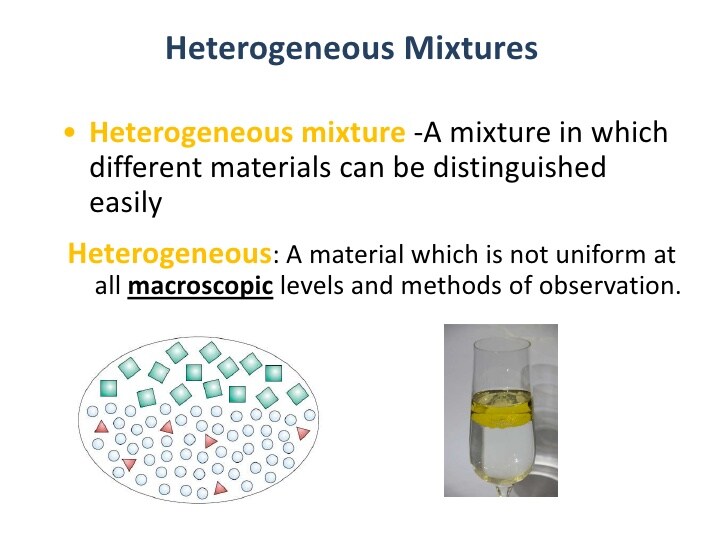
- Heterogeneous mixture: Not thoroughly blended, so you can see and pick out an individual part of the mixture.
Before really digging into these mixtures, let’s learn to say them correctly. These are five- and six-syllable words:
- Homogeneous: ho-mo-gee-nee-us
- Heterogeneous: het-er-oh-gee-nee-us
Both words are adjectives, not nouns. You have to use them to describe something else, like a mixture. You can’t say, “This is a heterogeneous.”
The prefix “homos” means “same,” while the prefix “hetero” means “different.” The root of “geneous” is a Greek word, “genos,” meaning a group, type, or stock. So, homogeneous means all the same group, and heterogeneous means all different groups together.
Think of two different bowls of soup: tomato soup is homogeneous, while the vegetable soup is heterogeneous.
Here are three more examples of heterogeneous mixtures:
- Students in a classroom
- A load of laundry
What Is A Mixture Definition Types Properties Examples

In our day to day life, we find a number of products labelled as pure. You would have noticed it on the packs of milk, butter, ghee etc. What does the term pure mean for us? For a common man, anything that is free from adulteration or any foreign substances can be termed as pure but from a scientific point of view, it is not true. In Science, anything that is made up of only a single element is known as pure. Thus for science, even though the pack of milk is labelled as pure, it is not so. It is not pure for science but it is considered to be a mixture. Let us look at the mixture and various types of mixtures in more depth.
Also Check: How To Avoid Parallax Error
Organic Chemistry What Is Reflux
January 17, 2015 By Sean Chua
We always see this reaction condition called Reflux when we study Organic Chemistry and their reactions.
I realised alot of students do not understand the true meaning of a reflux. This includes some of my JC2 and IB students who recently join our A-Level H2 Chemistry Tuition Classes.
So what really is Reflux?
Many organic chemical reactions take very long to complete, and in order to speed up these reactions, heat is applied. However, organic compounds are usually simple molecular structures with low boiling points. As such, most organic chemicals are quite volatile, and if heated they will evaporate and be lost. The solution to this problem is to heat the reaction mixture under reflux.
Diagram below shows the basic set-up of Refluxing:
Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a condenser. The vapours produced above the reaction continually undergo condensation, returning to the flask as a condensate.
The reactants for reflux experiments can be solid and liquid, or both liquids.
The condenser is always completely filled with water to ensure efficient cooling.
The vapours, which are given off from the liquid reaction mixture, change from gas phase back to liquid phase due to heat loss. This then causes the liquid mixture to fall back into the round bottom flask.
Whats The Best Way To Eat A Bag Of Jelly Beans
Whats the best way to eat a bag of jelly beans?
Many people open the bag and eat all the candy, no matter what flavor each piece is. Others pick through the collection. They might say I dont like the orange ones. Or maybe they just care for the lemon ones. There are different kinds of jelly beans in the mixture and people will eat what they want and get rid of the rest.
Read Also: What Is The Molecular Geometry Of Ccl4
Examples Of Mixture In A Sentence
mixturemixturemixturemixture BGRmixture baltimoresun.commixture Bon Appétitmixture San Diego Union-Tribunemixture ForbesmixtureThe Arizona Republicmixture Dallas Newsmixture CNN
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘mixture.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
For A Gaseous Mixture
The activity is of the form:
where i is the dimensionless fugacity coefficient of the species i, xi the mole fraction of the species in the gaseous mixture and p the pressure expressed in bar.
The quantity fi has the dimension of pressure and is fugacity of the gas: for a pure Ideal gas, the fugacity coefficient is equal to one.
The value p0 is the standard atmospheric pressure. It is equal to 1 bar and is present to cancel the dimensions of the formula.
Don’t Miss: What Is Elastic Force In Physics
What Is A Mixture In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A mixture is what you get when you combine two substances in such a way that no chemical reaction occurs between the components, and you can separate them again. In a mixture, each component maintains its own chemical identity. Typically mechanical blending combines components of a mixture, although other processes may produce a mixture .
Technically, the term “mixture” is misused when a recipe calls for you to mix, for example, flour and eggs. A chemical reaction does occur between those cooking ingredients. You can’t undo it. However, mixing dry ingredients, such as flour, salt, and sugar, does produce an actual mixture.
Even though the components of a mixture are unchanged, a mixture may have different physical properties than either of its components. For example, if you combine alcohol and water, the mixture has a different melting point and boiling point than either component.
Homogeneous And Heterogeneous Mixtures
Mixtures can be either homogeneous or heterogeneous: a mixture in which constituents are distributed uniformly, such as salt in water, is called homogeneous, whereas a mixture whose constituents are clearly separate from one another, such as sand in water, it is called heterogeneous.
In addition, “uniform mixture” is another term for homogenous mixture and “non-uniform mixture” is another term for heterogeneous mixture. These terms are derived from the idea that a homogeneous mixture has a uniform appearance, or only one visible phase, because the particles are evenly distributed. However, a heterogeneous mixture has non-uniform composition and its constituent substances are easily distinguishable from one another .
Several solid substances, such as salt and sugar, dissolve in water to form a special type of homogeneous mixture called a solution, in which there is both a solute and solvent present. Air is an example of a solution as well: a homogeneous mixture of gaseous nitrogen solvent, in which oxygen and smaller amounts of other gaseous solutes are dissolved. Mixtures are not limited in either their number of substances or the amounts of those substances, though in a homogeneous mixture the solute-to-solvent proportion can only reach a certain point before the mixture separates and becomes heterogeneous.
In physical chemistry and materials science, “homogeneous” more narrowly describes substances and mixtures which are in a single phase.
Read Also: Fsa Algebra 1 Eoc Practice
What Are The Classifications Of Mixtures
Mixtures are classified as heterogeneous or homogeneous and further classified due to the particle size of the components or substances. These classifications are a solution, a colloid and a suspension.
A solution has tiny particles sizes that are less than 1 nanometer in diameter. Components of a solution can’t be separated by centrifuging or decanting the mixture. An example of this is air.
A colloid mixture looks homogeneous without magnification, but when you see it under a microscope, you can see that it’s not mixed evenly. Particle sizes of colloids are from 1 nanometer to 1 micrometer. The separate substances in a colloid can be isolated by a centrifuge. An example of a colloid is hair spray where the liquid is an aerosol that combines with a gas.
A suspension has larger particles than the above two mixtures. At times, the mixture appears heterogeneous. Suspensions have stabilizing agents to keep the particles from separating naturally from each other. Both decantation and centrifugation can separate suspensions. An example of a suspension is salad dressing with vinegar and water. The heavier substance of the oil separates and goes to the bottom of the container while the water floats on top.
Related Articles
Where Does Heterogeneous Come From
The first record of heterogeneous comes from around 1620. It comes from the Greek heterogens, from hetero, meaning different, and génos, kind.
In general, things that are homogeneous are all the same, and things that are heterogeneous consist of a variety of different parts. The same thing goes in chemistry. Homogenous mixtures are uniform in consistency. But the different elements of a heterogeneous mixture, such as ice floating in water, are often easy to distinguish and even sometimes separate.
Recommended Reading: Who Are Paris Jackson’s Biological Parents
Is A Pencil A Mixture Or Compound
Aluminum: element carbon dioxide: compound sugar and water: solution, homogeneous mixture sulfuric acid: compound an orange: heterogeneous mixture a pencil: heterogeneous mixture nitrogen: element gasoline: solution, homogeneous mixture raisin bread: heterogeneous mixture water: pure, compound, tap water.
Mixtures Are Unlike Chemical Compounds Because:
- The substances in a mixture can be separated using physical methods such as filtration, freezing, and distillation.
- There is little or no energy change when a mixture forms.
- Mixtures have variable compositions, while compounds have a fixed, definite formula.
- When mixed, individual substances keep their properties in a mixture, while if they form a compound their properties can change.
Read Also: What Is Abiotic In Biology
Chemically Pure And Isotopically Pure
Chemists and nuclear scientists have different definitions of a pure element. In chemistry, a pure element means a substance whose atoms all have the same atomic number, or number of protons. Nuclear scientists, however, define a pure element as one that consists of only one stable isotope.
For example, a copper wire is 99.99% chemically pure if 99.99% of its atoms are copper, with 29 protons each. However it is not isotopically pure since ordinary copper consists of two stable isotopes, 69% 63Cu and 31% 65Cu, with different numbers of neutrons. However, a pure gold ingot would be both chemically and isotopically pure, since ordinary gold consists only of one isotope, 197Au.
How Do You Like Your Coffee
How do you like your coffee?
Many people enjoy a cup of coffee at some point during the day. Some may drink it black, while others may put cream and sugar in their coffee. You can buy high-end coffee drinks at espresso stands . Whatever your preference, you want the coffee to be the same at the beginning and the end of your drink. You dont want the components to separate out, but you want your drink to be uniform from top to bottom.
Don’t Miss: Which Founding Contributors To Psychology Helped Develop Behaviorism
Properties Of Homogeneous Mixtures
Homogeneous mixtures have several identifying properties:
Saltwater is an example of a homogeneous mixture that can be easily separated by evaporation.
Many countries use evaporation to capture pure drinking water from seawater, leaving behind salt that can be sold for profit.
Sugar water is another example of a homogeneous mixture that can be separated by evaportion.
Origin Of The Elements
Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons. .
The 94 naturally occurring chemical elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the Big Bang. This Big Bang nucleosynthesishappened only once the other processes are ongoing. Nuclear fusion inside stars produces elements through stellar nucleosynthesis, including all elements from carbon to iron in atomic number. Elements higher in atomic number than iron, including heavy elements like uranium and plutonium, are produced by various forms of explosive nucleosynthesis in supernovae and neutron star mergers. The light elements lithium, beryllium and boron are produced mostly through cosmic ray spallation of carbon, nitrogen, and oxygen.
In addition to the 94 naturally occurring elements, several artificial elements have been produced by human nuclear physics technology. As of 2021, these experiments have produced all elements up to atomic number 118.
Recommended Reading: What Is The Molecular Geometry Of Ccl4
Examples That Are Not Mixtures
Just because you mix two chemicals together, don’t expect you’ll always get a mixture! If a chemical reaction occurs, the identity of a reactant changes. This is not a mixture. Combining vinegar and baking soda results in a reaction to produce carbon dioxide and water. So, you don’t have a mixture. Combining an acid and a base also does not produce a mixture.
Discovery And Recognition Of Various Elements
Ten materials familiar to various prehistoric cultures are now known to be chemical elements: Carbon, copper, gold, iron, lead, mercury, silver, sulfur, tin, and zinc. Three additional materials now accepted as elements, arsenic, antimony, and bismuth, were recognized as distinct substances prior to 1500 AD. Phosphorus, cobalt, and platinum were isolated before 1750.
Most of the remaining naturally occurring chemical elements were identified and characterized by 1900, including:
- The more common radioactive elements, including uranium, thorium, radium, and radon
Elements isolated or produced since 1900 include:
- The three remaining undiscovered regularly occurring stable natural elements: hafnium, lutetium, and rhenium
- Plutonium, which was first produced synthetically in 1940 by Glenn T. Seaborg, but is now also known from a few long-persisting natural occurrences
- The three incidentally occurring natural elements , which were all first produced synthetically but later discovered in trace amounts in certain geological samples
- Four scarce decay products of uranium or thorium, , and
- Various synthetic transuranic elements, beginning with americium and curium
You May Like: How To Find Ksp
What Does Heterogeneous Mean
Heterogeneous most generally means consisting of different, distinguishable parts or elements.
The word is used in a more specific way in the context of chemistry to describe a mixture consisting of two or more different substances or the same substance in different phases of matter .
In either sense, the state of being heterogeneous is heterogeneity.
The general sense of heterogeneous is not as commonly used as the general sense of its opposite, homogeneouswhich most often means consisting of parts or elements that are all the same. The word homogenous can be used to mean the same thing.
The word heterogenous is very similar in spelling but not in meaning. Its used in the context of biology and medicine to refer to something that originated outside of the body or that is derived from another individual or species .
Example: The exhibit features a heterogeneous mix of artifacts from different cultures and eras.