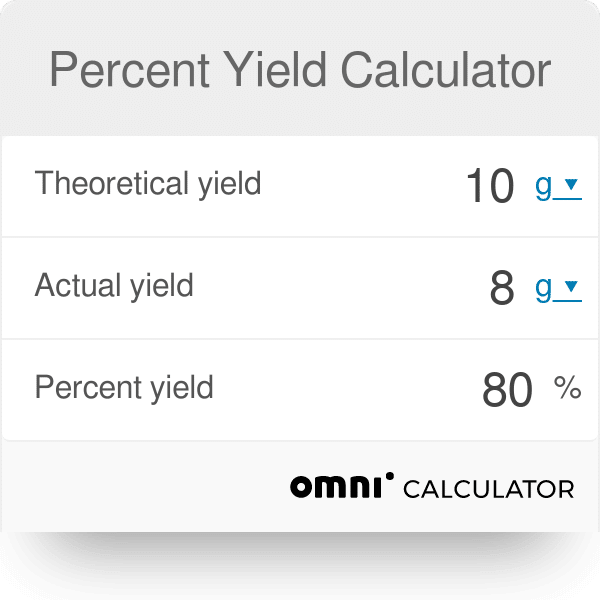
Calculate The Percent Yield
Remember the difference between percent yield and theoretical yield. Percent yield is the amount of product that is produced in a chemical reaction, as opposed to the amount that was theoretically possible. To calculate percent yield, simply divide the actual yield by the theoretical yield and multiply by 100. See the following formula:
How To Calculate Percent Yield In Chemistry In 10 Steps
To learn how to calculate percent yield in chemistry, lets review an example:
Azobenzene has the formula C6H5N and is a photoswitchable chemical. Azobenzene-based compounds are used in the textile industry as colorants and dyes.
What is the theoretical yield, percent yield, and actual yield of the following reaction if 0.10L of nitrobenzene , 0.30L of triethylene glycol yields 55g of azobenzene?
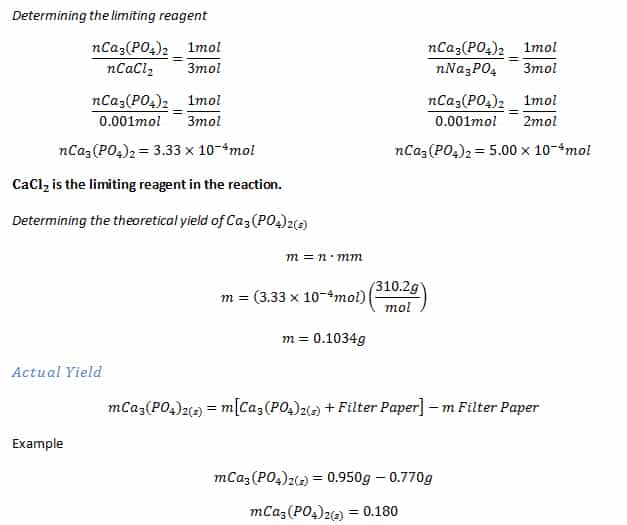
The Limiting Reagent And Excess Reagent
In a chemical reaction, you have both limiting reagents and excess reagents. The limiting reagents are the lesser in numbers and determine how much of a product will result. Therefore, limiting reagents often equals product. Excess reagents are the chemical or chemicals left over, the by-products of a reaction. The limiting reagent is important because for higher effectivity you want a balanced reaction. The ratio of limiting reagent, or limiting reactant and excess reagent, should be as close to 1:1 as possible. The more balanced the solution, the less waste results.
Also Check: What Is Unit In Chemistry
Multiply By 100 To Convert To A Percentage
To find the percent yield and calculate a full percentage, take the decimal results from the above step and multiply them by 100. This is the percent yield of a chemical reaction and helps to make the most product with the least waste while serving to indicate the efficiency of the method.
Read more:15 Top Chemistry Degree Jobs
Calculating Theoretical Yield And Percent Yield Adipic
Chemistry: A Molecular Approach | 3rd Edition
Calculating the Theoretical Yield and the Percent Yield for a Reaction
Adipic acid, \, is used to produce nylon, The acid is made commercially by a controlled reaction between cyclohexane \\) and \:
\+5\mathrm_2\rightarrow2\ \mathrm_2\mathrm_6\mathrm_8\mathrm_4+2\mathrm_2\mathrm\)
Assume that you carry out this reaction with 25.0 g of cyclohexane and that cyclohexane is the limiting reactant. What is the theoretical yield of adipic acid?
If you obtain 33.5 g of adipic acid from your reaction, what is the percent yield of adipic acid?
Accepted Solution
Recommended Reading: What Is The Rdw Process In Math
Why Is Percent Yield Less Than 100%
Due to loss and gain of heat subject to the environment, atmospheric pressure and humidity, incomplete reactions and loss of sample during an experiment, the actual yield is usually less than the theoretical yield. This is why the percent yield is less than 100%.In rare cases, the percent yield may be more than 100%. In these cases, the recovered sample is more than that is calculated theoretically.If the actual and theoretical yields are same, the ratio will be 1:1 and hence, the percent yield will be 100%. The higher the percent yield, the better the results of an experiment.
Calculating The Percent Yield Of Alum
To determine the percent yield of a product in a chemical reaction we need to know the amount of all reactants used, the amount of the product formed and the balanced chemical reaction. From the balanced chemical reaction and the amount of reactants, we determine first the limiting reagent and then theoretical yield of the product. The percent yield is then simply the actual amount of product obtained divided by the theoretical yield times 100.
For the reaction of Al with KOH to form alum the balanced chemical reaction is as follows:
2 Al + 2 KOH + 22 H2O + 4 H2SO4 2 KAl2 + 12 H2O + 3 H2
To simplify things we have told you that the Al is the limiting reagent . Since we do not need to determine the limiting reagent, our first step is to determine the amount of alum that can theoretically be formed from the amount of Al that we have. Lets assume that we used 0.475 g of Al and that we obtained 2.930 g of alum. Note that throughout this page extra insignificant figures carried along in the calculation to prevent rounding errors.
First, we must determine the moles of Al in 0.475 g of Al. You should get 0.01750 moles of Al .
Now convert moles of Al to moles of alum using the stoichiometric factor from the balanced chemical equation. You should have found that the reaction could form 0.01750moles of alum .
Determine the percent yield. Your result should be 35.1% to the correct number of significant figures, although this would often only reported as 35% .
Don’t Miss: How To Calculate Average Uncertainty In Physics
Calculating Percent Yield Example
Now that we know the steps to calculate percent yield, lets walk through an example:
Use the balanced chemical reaction below. If 40.00 g of Acetylene and 65.00 g of Oxygen are used, and 25.00 g of water are produced, what is the percent yield?
First step is to find limiting reagent& theoretical yield of water:
Using dimensional analysis on both reagents, acetylene is found to produce a lower amount of product than oxygen because of this acetylene is our limiting reagent.
27.67g is our theoretical yield. The last step is to plug our numbers into the percent yield equation.
Our percent yield is 90.35%.
For more example questions to try, click here!
How To Calculate Percentage Yield
Percentage yield is the weighted average of the product obtained divided by the theoretical yield. The quantity of product received from a chemical process is called the actual yield, whereas the quantity of product produced from the stoichiometric or balanced equation when the limiting reactant is used is called the theoretical yield. For a chemical reaction, the actual and theoretical yields are measured in the same units. The most commonly used units for these quantities are moles or grams.
Percentage yield Formula
The ratio of experimental yield to the theoretical yield multiplied by 100 gives the percentage yield of a chemical reaction. Generally, the value of percentage yield is less than 100 percent since the actual yield produced after the reaction is frequently smaller than the theoretical value. If its value is more significant than 100%, it clearly indicates that more sample was retrieved from the reaction than expected. If the actual and theoretical yields are equal, the percent yield is 100%.
P = × 100 %
- P is the percentage yield,
- A is the actual yield,
- T is the theoretical yield.
For example,
=> T = × 100
=> T = 2.7 moles
Problem 7: Calculate the percentage yield for the below decomposition reaction if the number of reactant moles is 6 and the actual yield is 2.8 moles.
2NO N2 + O2
Solution:
Recommended Reading: What Is Sample Space In Math Terms
Calculate Molar Mass Of Reactants
Molar mass is the mass of one mole of a substance. It’s also known as molar weight and can be calculated using average atomic masses on the periodic table. Use the below atomic masses taken from the periodic table to calculate the molar mass of each reactant rounded to 1 decimal point.
|
Element |
- n = number of moles
In the example, we have 0.10L of nitrobenzene. Convert this to moles using a line equation using the given density .
We also have 0.30L of triethylene glycol. Convert this to moles with a line equation using the given density.
Why Is Percent Yield Important
While the percent yield formula is used to better understand chemical reactions, its also valuable for other reasons, such as finances. A company that creates a chemically-based product uses the percent yield formula to evaluate their finances and productivity in product creation.
A low percent yield describes a reaction that is extremely far off from the expected result. That means that the production is being completed inefficiently. A lack of productivity destroys even the strongest companies because it wastes money and time.
You May Like: What Is Cristae In Biology
What Are Limiting Reactants
Sometimes we do not have enough of a reactant to form the amount of product we need.
Imagine you make nine cupcakes for a party but eleven guests show up. You should have made more cupcakes! Now the cupcakes are a limiting factor.
Fig. 2 – Limiting reactant
In the same way, if you do not have enough of a certain reactant for a chemical reaction, the reaction will stop when the reactant is all used up. We call the reactant a limiting reactant.
A limiting reactant is a reactant that is all used up in a chemical reaction. Once the limiting reactant is all used up, the reaction stops.
One or more of the reactants may be in excess. They are not all used up in a chemical reaction. We call them excess reactants.
Compare Ratios To Find The Limiting Reactant
A limiting reactant is a substance that becomes exhausted first during a chemical reaction. It’s important to identify the limiting reactant in a reaction because it will determine the maximum yield that can be achieved.
In other words, knowing the limiting reactant allows you to calculate how much of a product can be produced from a given amount of starting material.
We can solve the limiting reactant problem by finding how many moles of the desired product that each of the reactants can produce. In this example, its:
In the sample problem, the limiting reactant is ni trobenzene and as the ratio of reaction is lower, there will be excess triethylene glycol .
Also Check: What Is K Space In Solid State Physics
How To Find/calculate Theoretical Yield
Its helpful to know how much product will be created with given quantities of reactants before initiating chemical reactions, known as the theoretical yield. This is an approach to employ when determining the theoretical yield of a chemical process. The same technique can determine how much of each reagent is needed to make a certain amount of product.
Formula To Calculate Percent Yield
The formula to calculate the percent yield is:
Percentage Yield = × 100 %
where,
- Actual yield – it gives the amount of product obtained from a chemical reaction
- Theoretical yield – it gives the amount of product obtained from the stoichiometric or balanced equation, using the limiting reactant to determine the product
- Units for both actual and theoretical yield need to be the same
You May Like: Introductory Algebra 2nd Edition Miller
Determine The Actual Yield Of Experiment
The actual yield is the amount of product obtained from a chemical reaction. The actual yield is obtained experimentally. The example problem states that the actual yield is 55g of azobenzene.
When not performing an experiment, the actual yield should be provided in the problem. Be sure to convert the actual yield to grams.
Limiting Reactant And Percent Yield
The limiting reactant of a balanced chemical equation is identified to determine theoretical yield. Because the limiting reactant isnt found in abundance, the reaction cant continue once used up.
To find the limiting reactant, the below points are to be remembered:
- If the quantity of reactants is given in moles, convert the results to grams.
- In grams per mole, divide the mass of the reactant by its molecular weight.
- Alternatively, we can multiply the amount of a reactant solution in millilitres by its density in grams per millilitre for a liquid solution. Then divide the result by the molar mass of the reactant.
- Multiply the mass obtained by the number of moles of the reactant in the balanced equation using either technique.
- Now we know how many moles each reactant has. To determine which is accessible in excess and which will be used up first , compare this to the molar ratio of the reactants.
To calculate the percent yield, first establish the amount of product that should be produced using stoichiometry. This is the maximum amount of product made with reactant amounts available. When a reaction is carried out in the lab, the actual yield is the amount of produced product. The percent yield is the percentage difference between the actual and theoretical yields.
Percent Yield = Mass of Actual Yield / Mass of Theoretical Yield x 100 percent
Also Check: Which Professionals Most Directly Use Geometry In Their Work
Yield Calculations Chemistry Tutorial
- Yield is the mass of product formed in a chemical reaction.
- Actual yield is the mass of product formed in an experiment or industrial process.
- Theoretical yield is the mass of product predicted by the balanced chemical equation for the reaction.
- Percentage yield = × 100
- Optimum yield is the best possible yield achieved for a set of given reaction conditions.
- For a chemical reaction which goes to completion:
- For a chemical reaction at equilibrium:
- For a chemical reaction at equilibrium, actual yield can be affected by factors such as:
actual yield = theoretical yield
actual yield < theoretical yield
temperature
pressure and volume
Please do not block ads on this website. No ads = no money for us = no free stuff for you!
Find The Ideal Ratio Of Reaction
The ideal ratio of reaction refers to how many moles of each reactant are required for the desired product. In this example, 2 moles of nitrobenzene reacts with 4 moles of triethylene glycol to produce 2 moles of azobenzene 2) and 4 moles of kethoxal and 1 mole of water, also called dihydrogen monoxide .
You May Like: What Is Sugar Classified As In Chemistry
What Is Theoretical Yield
The theoretical yield is the maximum amount of product that can be obtained from a chemical reaction. It is calculated based on the stoichiometry of the reaction, which is the study of the proportions of the reactants and products in a chemical reaction. The theoretical yield is determined by using the balanced chemical equation for the reaction and the known amounts of the reactants.
For example, if you have 100 grams of reactant A and 200 grams of reactant B, and the balanced chemical equation for the reaction is A + B > C, then the theoretical yield of product C would be 200 grams, because that is the maximum amount that could be produced from the given amount of reactants. In reality, however, the actual yield of a reaction is often less than the theoretical yield, due to a variety of factors such as incomplete conversion of the reactants or loss of product during the reaction.
How To Find The Limiting Reactant

To figure out which of the reactants in a chemical reaction is the limiting reactant, you must start with the balanced equation for the reaction, then work out the relationship of the reactants in moles or by their mass.
Let’s use an example to find the limiting reactant in a chemical reaction.
$$ C_2H_4 + Cl_2\rightarrow C_2H_4Cl_2 $$
The balanced equation shows 1 mole of ethene reacts with 1 mole of chlorine to produce 1 mole of dichloroethane. Ethene and chlorine are all used up when the reaction stops.
\begin & C_2H_4 +Cl_2\rightarrow C_2H_4Cl_2\\ \text \qquad & 1mole\quad 1mole\\ \text\qquad & 0 moles\quad 0moles\quad 1mole\end
What if we use 1.5 moles of chlorine? How much of the reactants are left over?
\begin & C_2H_4 \space +\space Cl_2\rightarrow \quad C_2H_4Cl_2\\ \text \qquad & 1mole\quad 1.5moles\\ \text\qquad & 0 moles\quad 0.5moles\quad 1mole\end
1 mole of ethene and one mole of chlorine react to make 1 mole of dichloroethane. 0.5 moles of chlorine is left over. Ethene is the limiting reactant in this case as it is all used up at the end of the reaction.
You can also use the trick of dividing the number of moles of each reactant by its stoichiometric coefficient to determine which reactant is limiting. The reactant with the smallest mole ratio is limiting.
For the above example:
Stoichiometric coefficient of \ = 1
Number of moles = 1
Stoichiometric coefficient of \ = 1
Number of moles = 1.5
1 < 1.5, therefore,\ is the limiting reactant.
Also Check: What Is Instinct In Psychology
How To Calculate The Percentage Yield For A Reaction With Excess Reagent
A chemist mixed $12~\mathrm$ of phosphorus with $35.5\ \mathrm$ of chlorine gas to synthesize phosphorus chloride . The yield was $42.4~\mathrm$ of $\ce$. The equation is $$\ce$$ Calculate the percentage yield.
I think that the amount of phosphorus the chemist used is in excess. I figured that only 1/3 of the amount of phosphorus the chemist used reacted to produce phosphorus trichloride. But the question didn’t mention anything about products in excess that is why I am not so sure of my answer, which is $92.51$.
First I found the amount of substance of phosphorus used which is $12/31$. Then I calculated the amount of substance of chlorine gas used . But $12/31\ \mathrm$ of $\ce$ needs $1.5 \times \ \mathrm$ of chlorine gas, so $\ce$ is in excess. The amount of substance of $\ce$ that will react will be $$0.5/1.5 = 1/3$$ Now $1/3\ \mathrm$ of $\ce$ produces $1/3\ \mathrm$ of $\ce$ and the molecular mass of $\ce$ is $137.5$ so the mass that is supposed to be produced is $137.5 \times 1/3$. Then I divided $42.4$ by $$ and multiplied by $100$.
The yield is typically calculated according to equation $$.
$$\text = \frac}}\tag$$
So for any reaction, you need to figure out:
- the reaction equation
- the amount of each reactant substance involved
- the amount of desired product theoretically possible by stoichiometric coefficients
- the actual amount of desired product.
The first is rather easy, especially since the equation $$ has been given previously.
$$\ce\tag$$
Theoretical Yield Quick Review
- Balance your equations.
- Find the mole ratio between the reactant and the product.
- Calculate using the following strategy: Convert grams to moles, use the mole ratio to bridge products and reactants, and then convert moles back to grams. In other words, work with moles and then convert them to grams. Don’t work with grams and assume you’ll get the right answer.
For more examples, examine the theoretical yield worked problemand aqueous solution chemical reaction example problems.
Recommended Reading: Where Can I Take The Gre Psychology Test