Activity Series For Cation Replacement In Single
- Li
Answer
Mg32 and Al
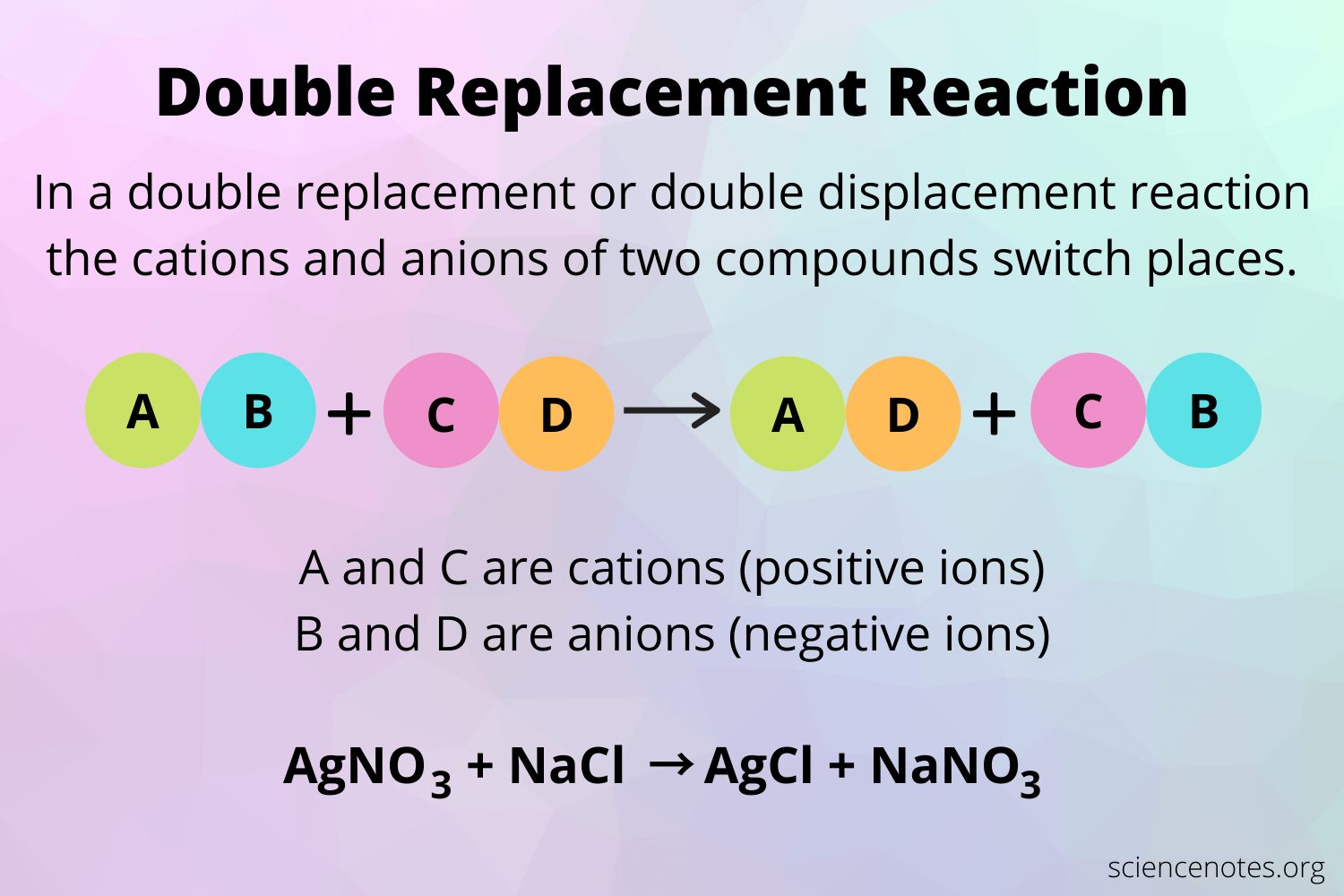
A double-replacement reaction occurs when parts of two ionic compounds are exchanged, making two new compounds. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products. An example is
CuCl2 + 2 AgNO3 Cu2 + 2 AgCl
There are two equivalent ways of considering a double-replacement equation: either the cations are swapped, or the anions are swapped. Either perspective should allow you to predict the proper products, as long as you pair a cation with an anion and not a cation with a cation or an anion with an anion.
What Occurs When The Products Have Less Enthalpy Than The Reactants
In exothermic reactions, the products have less enthalpy than the reactants, and as a result, an exothermic reaction is said to have a negative enthalpy of reaction. This means that the energy required to break the bonds in the reactants is less than the energy released when new bonds form in the products.
Health Safety And Technical Notes
- Copper sulfate solution, CuSO4 see CLEAPSS Hazcard HC027c.
- Lead nitrate solution, Pb2, see CLEAPSS Hazcard HC057a.
- Magnesium sulfate solution, MgSO4 see CLEAPSS Hazcard HC059c.
- Zinc sulfate solution, ZnSO4 see CLEAPSS Hazcard HC108b.
- Copper foil, Cu see CLEAPSS Hazcard HC026.
- Magnesium ribbon, Mg see CLEAPSS Hazcard HC059a. Do NOT leave in a place where pupils would have potentially unsupervised access.
- Zinc foil, Zn see CLEAPSS Hazcard HC017.
- Lead foil, Pb, see CLEAPSS Hazcard HC056.
Also Check: Geopolitics Definition Ap Human Geography
What Is Reactivity Series
Reactivity series is the series of metals based on their reactivity from highest to lowest. So, reactivity series of metals can be defined as a series of metals, in order of reactivity from highest to lowest. It is also known as activity series. The reactivity of metals is because of their incomplete outer orbitals or due to their electronic configuration. Metals form positively charged ions as they tend to lose electrons. Metals with high atomic numbers tend to be more reactive as their electrons are far from the positively charged nucleus. So, they can be removed easily.
We’ve Displaced Our Brains
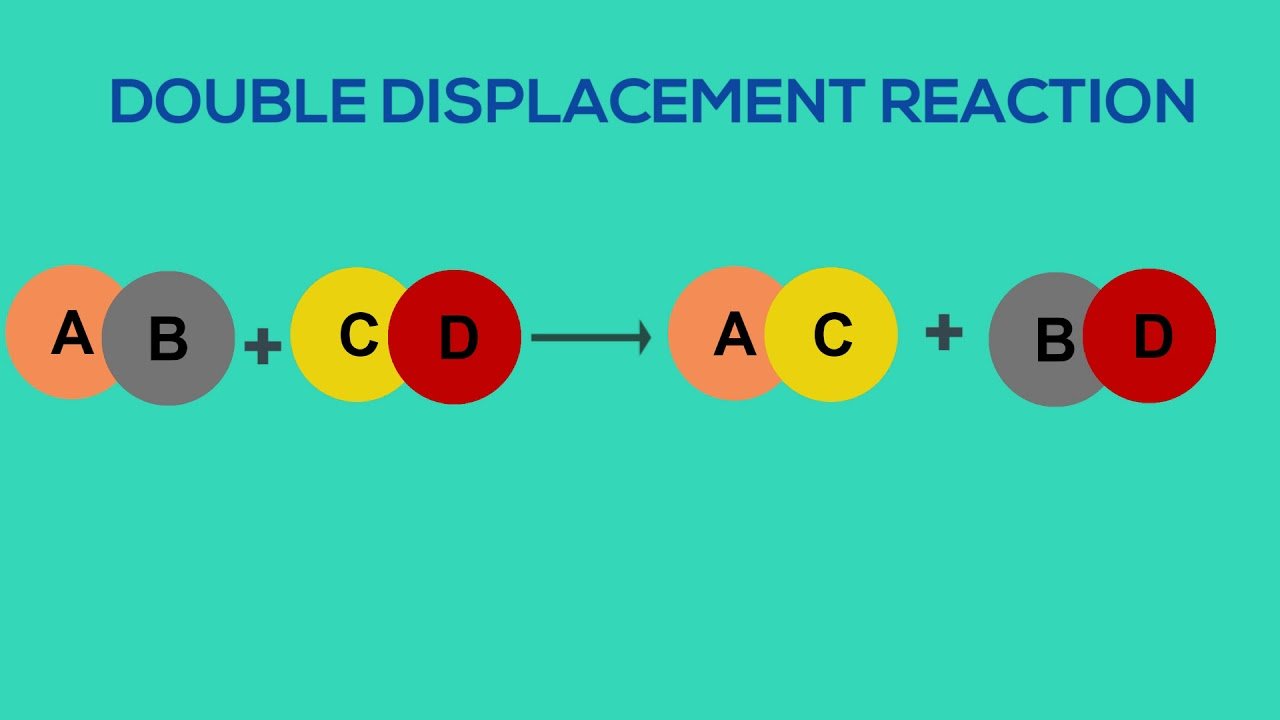
A single displacement reaction is exactly how to sounds, one element in a compound is displaced and replaced by a different element. There are several types of single displacement reactions. The general form of a single displacement reaction is:
AB + C AC + B
In ametal displacement reaction, a more active metal kicks out a less active metal from a compound. Talk about elemental unfriendliness. For example, a big bad iron atom will displace copper from a copper sulfate molecule because iron is more reactive than copper. Poor copper is left to fend for itself.
Fe + CuSO4 Cu + FeSO4 .
Just so we’re all on the same page, the “aq” means aqueous, or the compound is dissolved in water.
Guessing which metals are the bigger bullies requires some detective work. Never fear, scientists have created a useful table called an “activity series.” It’s a ranking of metals from most active to least active. Remember: more active metals will kick out less active metals from compounds in a heartbeat.
The Activity Series of Common Metals
Hydrogen can also be displaced from compounds by all alkali metals and even some alkaline earth metals. Do you recall what happens when a chunk of sodium is thrown in water? An explosion occurs because when hydrogen is displaced from water, it quickly forms hydrogen gas. The reaction is also exothermic which causes the hydrogen gas to ignite.
2 Na + 2 H2O 2 NaOH + H2
Mg + 2 HCl MgCl2 + H2
F2> Cl2> Br2 > I2
Cl2 + 2 NaI 2 NaCl + I2
AB + CD AC + BD
Recommended Reading: Ccl4 Vsepr
What Is Displacement In Chemistry
A displacement reaction is a reaction where a part of the reactant replaces another reactant. It is also known as the metathesis or replacement reaction.
The displacement reactions involve metal and a compound of a different metal. Talking about it, in a displacement reaction, A more reactive metal will displace a less reactive metal from its compounds.
A displacement reaction is easily seen when the salt of less reactive metal is in the solution. During the reaction, the more reactive metal gradually disappears as it forms a solution, whereas the less reactive metal coats the surface of the more reactive metal.
Book Your 60-minutes Free Trial class NOW!
Single And Double Displacement Reactions
- ONLINE CHEMISTRY LAB MANUAL at Santa Monica College
- To perform and observe the results of a variety of single and double displacement reactions,
- To become familiar with some of the observable signs of these reactions,
- To identify the products formed in each of these reactions,
- To write balanced chemical equations for each single and double displacement reaction studied.
During a chemical reaction both the form and composition of matter are changed. Old substances are converted to new substances, which have unique physical and chemical properties of their own. Some of the observable signs that a chemical reaction has occurred include the following:
- A metallic deposit appears
- A color change occurs
- A precipitate appears
Note that there are many other observable signs for chemical reactions, but these are the ones most likely to be encountered in this lab.
Also Check: Who Is Paris Jackson Mother
Solubility Rules And Activity Series
Solubility Rules:
Activity Series
Materials and Equipment
Solids: Copper metal, zinc metal, magnesium metal, solid sodium bicarbonate
Solutions: 6 M sodium hydroxide, 6 M hydrochloric acid, 6 M ammonium hydroxide, 5% acetic acid all other solutions are 0.1 M and include silver nitrate, barium chloride, sodium sulfate, potassium chloride, lead nitrate, iron chloride, sodium carbonate, cobalt nitrate, sodium phosphate, zinc nitrate, copper sulfate, sodium chloride, potassium nitrate, nickel nitrate.
Equipment: 6 large test tubes, 8 small test tubes, plastic test tube rack
Safety
Be especially cautious when using the 6 M \ and 6 M \ as they can burn your skin. Also be aware that skin discoloration will result from contact with \. If you feel any tingling sensations or see any color changes on your skin, flush with water immediately for a minimum of 15 minutes. Inform your instructor of any chemical contact as soon as possible.
Solids Liquids & Gases
Often we write the state of a compound in a reaction using parenthesis and the letters , , & . Aqueous solutions are solutions in which water is the solvent. They are so ubiquitous in our chemistry that they get the special designation . Here’s the table above, rewritten with the added state information:
| Fe + O2 FeO2 | solid Iron combines with oxygen gas to form solid iron oxide |
| H2 + Br2 2 HBr | Hydrogen gas combines with bromine gas to form two molecules of gaseous hydrogen bromide |
| 2 NH3 + H2O + CO2 2CO3 | Two molecules of gaseous ammonia, a liquid water molecule and a molecule of carbon dioxide gas combine to form a molecule of aqueous ammonium carbonate. |
Aqueqous solutions are mixtures of soluble ionic or nonionic compounds and water. They are pure substances.
Recommended Reading: Holt Geometry Answer Key
Examples Of Synthesis Reactions
Here are a few examples of synthesis reactions. Compare them to the model reaction above to make sure you get the idea. For each reaction there’s a version written in an English sentence on the right, so that you might be able to pick up on the meaning more easily:
| Reaction | |
|---|---|
| Diatomic hydrogen combines with diatomic bromine to form two molecules of hydrogen bromide | |
| 2 NH3 + H2O + CO2 2CO3 | Two molecules of ammonia, a water and a carbon dioxide combine to form a molecule of ammonium carbonate. |
| CaO + SO2 CaSO3 | Calcium oxide and sulfur dioxide react to form calcium sulfite. |
A note about the sentences above : These aren’t the only ways to describe these reactions, just ones I though appropriate at the moment. You might do a better job.
Synthesis reactions
In a synthesis reaction, two or more simpler components are joined to form a more complex compound.
Double Displacement Reaction Definition And Examples
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A double displacement reaction is a type of reaction in which two reactants exchange ions to form two new compounds. Double displacement reactions typically result in the formation of a product that is a precipitate.
Double displacement reactions take the form:AB + CD AD + CB
Recommended Reading: Ct Algebra 1 Curriculum Version 3.0
Examples Of Distance And Displacement
Question 1. John travels 250 miles to North but then back-tracks to South for 105 miles to pick up a friend. What is Johns total displacement?
Answer: Johns starting position Xi= 0.
Her final position Xf is the distance travelled N minus the distance South.
Calculating displacement, i.e.D.
D = 0
D = 145 mi N
Question 2. An object moves along the grid through points A, B, C, D, E, and F as shown below. The side of square tiles measures 0.5 km.
a) Calculate the distance covered by the moving object.
b) Find the magnitude of the displacement of the object.
Solution:
Read More:Difference Between Distance and Displacement
Watch and learn the laws of motion with the help of animations.
We, at BYJUS, strongly believe that a spirit of learning and understanding can only be inculcated when students are curious and that curiosity can be brought about by creative and effective teaching. It is this approach that makes our lectures so successful and gives our students an edge over their counterparts.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Displacement Reactions: Definition Types And Examples
Exams Prep Master| Updated On -Feb 13, 2022
Displacement Reaction refers to a chemical reaction wherein a more reactive element displaces a less reactive element from its compound. In other words, the atoms of the more reactive metal push their electrons on to ions of the less reactive metal. Both metals as well as non-metals participate in these displacement reactions. Here we will discuss the concept in detail with a few examples.
|
Table of Content |
A displacement reaction is a chemical reaction. It can be defined as a reaction where an atom or multiple atoms are exchanged in a compound.
A displacement reaction can be represented as:
X + Y-Z Z+ X-Y
In the given reaction, X replaces Z from the compound YZ.
The reaction takes place when a more reactive element displaces a less reactive element. The reactivity is decided by the reactivity series.
There are two types of displacement reactions:
- Single displacement reaction
- Double displacement reaction
Displacement reactions are beneficial and are used in many everyday activities. Some of the important uses of this reaction are electroplating, thermite welding, steel making, acid digestion etc.
Also Check: Density Vs Concentration Human Geography
Key Takeaways: Double Displacement Reaction
- A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
- Usually, a double displacement reaction results in precipitate formation.
- The chemical bonds between the reactants may be either covalent or ionic.
- A double displacement reaction is also called a double replacement reaction, salt metathesis reaction, or double decomposition.
The reaction occurs most often between ionic compounds, although technically the bonds formed between the chemical species may be either ionic or covalent in nature. Acids or bases also participate in double displacement reactions. The bonds formed in the product compounds are the same type of bonds as seen in the reactant molecules. Usually, the solvent for this type of reaction is water.
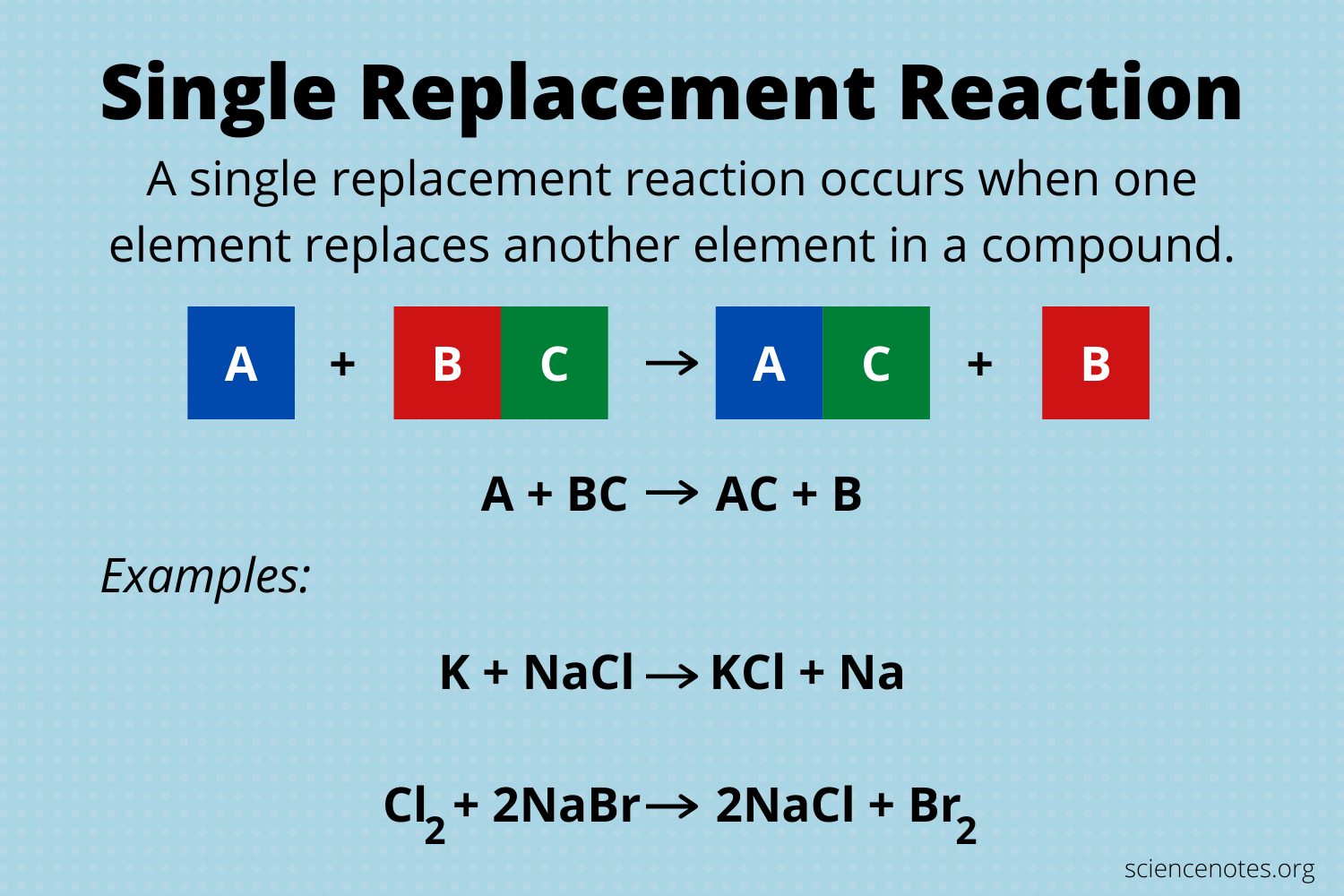
What Is Single Displacement Reaction
Those reactions in which one element replaces another element from its salt or compound are called single displacement reactions. These are also called single replacement reactions. General representation can be written as well
A + B-C A-C + B
It will occur if A is more reactive than B. Generally, metals and its salts give single displacement reactions. In these reactions more reactive metal displaces less reactive metal from its salt. For example, potassium is more reactive than magnesium, so potassium replaces magnesium from magnesium chloride. The reaction between potassium and magnesium chloride occurs as follows
2K + MgCl2 2KCl + Mg
Don’t Miss: How To Calculate Half Life Chemistry
Define Double Displacement Reaction
A double displacement reaction takes place when two iconic components of a compound switch positions with each other.
A double displacement reaction can be represented as follows:
AB + CD CB + AD
In the given representation, A has switched position with C and similarly C with A.
Examples of double displacement
- When sodium sulphate solution is mixed with barium chloride solution, white precipitation of barium sulphate is formed instantly.
BaCl2 + Na2SO4 BaSO4 + 2NaCl
- The reaction between silver nitrate and sodium chloride is also a double displacement reaction represented as
AgNO3 + NaCl à AgCl + NaNO3
Displacement Reactions Between Metals And Their Salts
Some metals are more reactive than others. In this experiment, a strip of metal is added to a solution of a compound of another metal. A more reactive metal displaces a less reactive metal from its compound. Students will investigate competition reactions of metals and determine a reactivity series of the four metals used
There are many ways of carrying out this series of reactions. The one described here uses a spotting tile but the same procedure could be adapted for use with test tubes. The advantages of the spotting tile method include:
- very small quantities of chemicals are used.
- the whole set of experiments is displayed together, making comparison easier.
- clearing-up afterwards is simple and avoids metal deposits being left in sinks.
Careful thought needs to be given to distribution of the chemicals to the class. Solutions could be distributed in test tubes, or in small bottles fitted with droppers for sharing between several pairs of students. Metals could be issued in sets. The teacher should keep control of the magnesium ribbon, dispensing short lengths when required.
There should be no flames alight so that students are not tempted to burn pieces of magnesium and the teacher should be alert to the possibility of pieces of magnesium being removed from the laboratory.
The experiment should take about 30 minutes.
You May Like: Geometry Escape Challenge A Answer Key
What Is Displacement Reaction Give An Example
A chemical reaction in which a more reactive element is displaced by the less reactive element from its compound is called a displacement reaction.
AB + C AC + B
When the iron is added to a copper sulphate solution, it displaces the copper metal
from copper sulphate solution to form iron sulphate and Copper.
Fe + CuSO4 FeSO4 + Cu
When zinc is added to a copper sulphate solution, it displaces the copper metal
from copper sulphate solution to form zinc sulphate and Copper.
Zn + CuSO4 ZnSO4 + Cu
Explore more such questions and answers at BYJUS.
Was this answer helpful?
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Don’t Miss: I Ready Answers Level E
Why Is Displacement Enthalpy
The heat of displacement is the heat change when one mole of a metal is displaced from its salt solution by a more electropositive metal. The thermochemical reaction for the displacement reaction of copper by zinc can be represented as follows. The heat of displacement of copper by zinc is -210 kJ mol-1.
Uses Of Displacement Reaction
A mixture of aluminium and ferric oxide is used to weld railway joints together.
The reaction is as follows:
2Al + Fe2O3 Al2O3 + 2Fe
Iron can be extracted from its ore by reaction with Carbon.
3C + 2Fe2O3 4Fe + 3 CO2
- Extraction of metals
A displacement reaction can also extract various other metals, such as chromium.
3C + 2Cr2O3 4Cr + 3CO2
- Acid neutralisation
Our stomach produces HCl acid, which leads to indigestion. Antacids consist of a base that leads to a displacement reaction.
Mg 2 + 2 HCl MgCl2 + 2 H2O
You May Like: Rationalizing Imaginary Denominators Worksheet Answers
What Is Water Displacement Definition
When an object enters water, it pushes out water to make room for itself. The object pushes out a volume of water that is equal to its own volume. This is called displacement. Any object that is in water has some buoyant force pushing up against gravity, which means that any object in water loses some weight.
Examples Of Combustion Reactions
| CH4 + 2O2 & #8652 CO2 + 2H2O | Methane gas is combusted in the presence of oxygen to yield carbon dioxide and water. |
| 2C2H5OC2H5 + 13O2 & #8652 8CO2 + 10H2O | Diethyl ether is burned in the presence of oxygen to yield carbon dioxide and water. |
| 2C6H6 + 15O2 & #8652 12CO2 + 6H2O | Two molecules of benzene are burned in the presence of excess oxygen to yield 12 molecules of CO2 and six molecules of H2O. |
Dimethyl ether, burned in the second reaction above, is an oxy-hydrocarbon. Think of it as a hydrocarbon that carries around a bit of its own oxygen for combustion. In fact, molecules like these can help to ensure that combustion reactions go to completion, especially in unfavorable conditions like cold weather. Diethyl ether is added to gasoline in some states in winter to help the gas combust fully, avoiding other products that are more harmful to life, such as carbon monoxide .
Recommended Reading: Linear Algebra What Is Span