Limitations Of Lewis Diagrams
Lewis diagrams are useful and accurate enough for determining the electron configuration of compounds composed out of main group elements, but they have some shortcomings. Not every chemical compound follows the exact bonding rules described above. As with any rule, there are exceptions. Transition metals, for instance, often dont follow the octet rule and instead fill their outer shell with 12 electrons. For some compounds, there is no one adequate diagram, so the electron configuration of those compounds are described as a hybrid of multiple Lewis diagrams. These are called;resonance structures.
We are like an atomic structure. Weve got a causal body thats linked together. Frederick Lenz
Lewis diagrams also do not give much information regarding the 3-dimensional geometric orientation of the atoms, which is important for explaining the polarity;and intermolecular bonding behavior of compounds. VESPR theory is a chemical modeling method that describes a molecules 3-dimensional shape and how that shape arises from the electrostatic repulsion of electron pairs. Electrons repel each other so a molecule tends to take a shape that minimizes the repulsion between electron pairs. This information can be used to predict the likely geometric structure of a given compound.
Ccl4 Molecular Geometry Or Shape
Another method we have is AXN for determining the molecular geometry of CCl4.;
Lets see how to use this method.
- A represents the central atom.
- X represents the bonded pairs of electrons to the central atom.
- N represents the lone pairs of electrons on the central atom
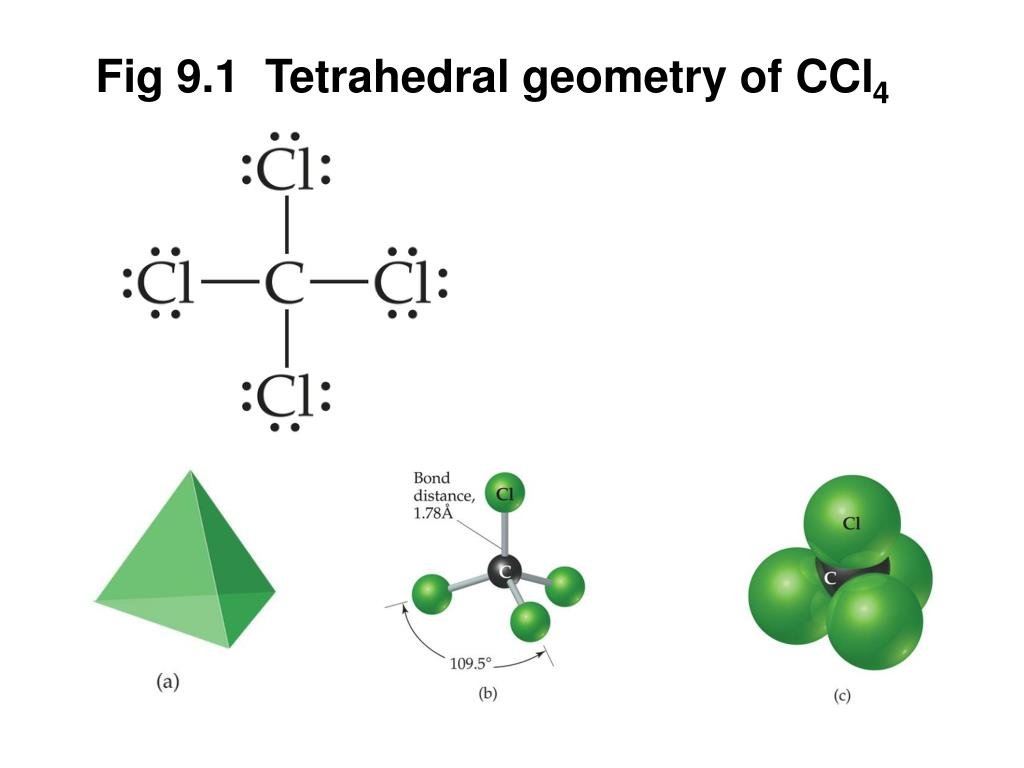
As per the CCl4 lewis dot structure, carbon is the central atom that has 4 bonded pairs of electrons and 0 lone pairs on it.
Hence formula of CCl4 becomes AX4.
According to the VSEPR chart, if any molecule represents the AX4; generic formula then the molecule and electron geometry of that molecule is tetrahedral.
| Bonded atoms | |
| T-shaped | Trigonal bipyramidal |
The bond angle of CCl4 is 109.5º because electrons around carbon will repel each other giving molecular geometry of CCl4 tetrahedral. Hence its bond angle is 109.5º approx.
What Is The Molecular Geometry Of Ccl4 What Is The Molecular Geometry Of Ccl4 Bent Linear Tetrahedral Trigonal Pyramidal Not E
Kp is defined for any reaction as:
aA +bB cC +dD
Kp is:
P in the equation is the partial pressure for each specie in the equilibrium.
We know the total pressure for the system at the beginning is 2 atm. We can say that at the beginning we have just so the total pressure for the system is also the partial pressure for ;
In the equilibrium we have the three species , and so now we need to estimate the partial pressure for each specie. ;
We know that the total pressure for the system in the equilibrium is 3.96 atm. The total pressure is equal to the sum of the partial pressure of each component in the mix as:
Until we reach the equilibrium its been used to produce and . We can call the among of ; that reacted until the system reach the equilibrium so in the equilibrium will be
When reviewing the molar ratio in the reaction we see that it is 1: 1 so the amount of that reacted will be equal to the amount of and
Don’t Miss: What Does Mole Mean In Chemistry
What Is The Formal Charge In Cf4 Lewis Structure And How To Calculate It
The formal charge of CF4 shows that which atom has a more positive or negative charge present on it.;
To calculate the formal charge in CF4 lewis dot structure. Use this equation given below:
Formal charge =
Now we will calculate the formal charge on carbon which is the central atom in the CF4 lewis structure.
Valence electron of carbon = 4
Non-bonding electrons on carbon = 0
Bonding electrons attached to carbon = 8; ; ; ; ; ;
- The total valence electron available for the CF4 lewis structure is 32.
- The hybridization number of CF4 is Sp³ and the steric number is 4.
- The bond angle of CF4 is 109.5º.
- CF4 is nonpolar in nature but the bond present in it is polar.
- The net dipole moment of carbon tetrafluoride is zero.
- The formal charge on carbon in CF4 is zero.
- Total 8 bonded electrons present in CF4 lewis dot structure.
- The molecular geometry of CF4 is tetrahedral and electron geometry is also tetrahedral.
What Is The Formal Charge In The Ccl4 Lewis Dot Structure And How To Calculate It
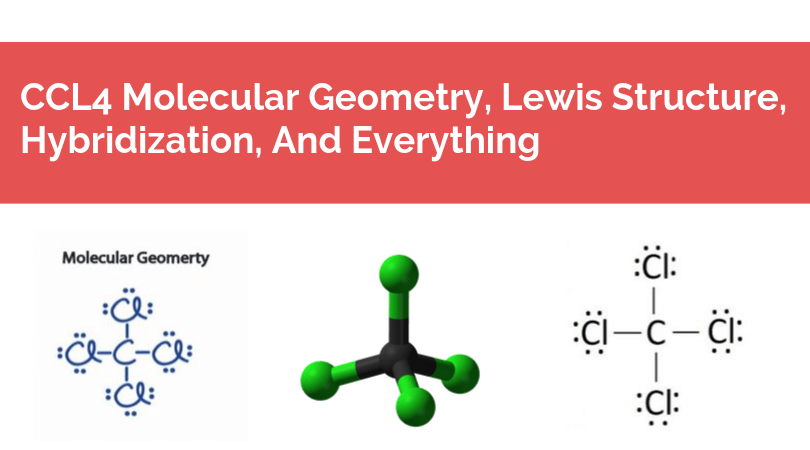
The formal charge in CCl4 shows that which atom has a more positive or negative charge present on it.
To calculate the formal charge in Carbon tetrachloride lewis structure, Use the given formula-
Formal charge =
Now we will calculate the formal charge on the central atom which is Carbon in the CCl4 Lewis structure.
Valence electron of Carbon = 4
Bonding electrons = 8
= 0 is the formal charge on the central atom.
Read Also: What Are Dyes In Chemistry
Connect Carbon And Chlorine With A Single Bond
Now we are going to start drawing the CCl4 lewis structure by connecting chlorine to carbon with the help of a single bond.
Now look at the above structure and find how many valence electrons we used till now. Valence electron means electrons that are shareable or transferable.
Hence, 4 single bonds are used in the above structure to connect chlorine with carbon and 1 single bond contains 2 electrons.
Therefore, 4 single bonds mean 8 electrons are used in the above structure from a total of 32 valence electrons.
= 24 valence electrons
So, we are left with 24 valence electrons more.
Find The Least Electronegative Atom And Placed It At Center
In this step, We will find the central atom to place in the lewis diagram by determining the least electronegative atom in between Carbon or Chlorine.
As electronegativity increase from left to right in the periodic table. Hence carbon is a less electronegative atom than chlorine. Therefore carbon is placed at the center and chlorine spaced evenly around it.
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
What Are The Various Names Of Ccl4
Carbon tetrachloride, a covalent compound, has multiple names based on its uses. A few of them are Halon-104, Freon 10, Refrigerant-10, Carbon Tet, Tetraform, methane tetrachloride, Tetrafinol, and benzinoform.
Carbon tetrachloride is a compound having the molecular formula CCl4. It consists of one carbon and four chlorine molecules. The carbon molecule is located at the center around which the four chlorine molecules are distributed.
Tetrachloromethane or carbon tetrachloride is known for its aromatic smell, which resembles that of chloroform. It is highly toxic in nature and has a high density value. This compound can be prepared by reacting methane with chlorine atoms. Further ahead, we explain the polarity values and Lewis dot structure of this compound.
What Is The Molecular Geometry Of Ccl4 Draw Its Vsepr And Lewis Structure
has a tetrahedral geometry with bond angles of 109.5 °.
Explanation:
Here are the steps that I follow when drawing a Lewis structure.
1. Decide which atom is the central atom in the structure.
That will be the least electronegative atom .
2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom a #”C”# #”Cl”# atoms attached to it.
3. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
4. Count the valence electrons in your trial structure .
5. Count the valence electrons you actually have available.
#”1 C + 4 Cl” = “1×4 + 4×7” = 32# .
6. The trial structure has exactly the same number of electrons as we have available.
Thus, the trial structure is the correct Lewis structure.
VSEPR Geometry
The Lewis structure shows that there are four electron regions about the central carbon atom.
The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible.
For these clouds in #”CCl”_4# to be as far as possible from one another, they will point toward the corners of a tetrahedron.
The bonds will emanate from the central atom at angles of 109.5 ° to each other.
The structure will be tetrahedral.
Don’t Miss: Practice 2 4 Reasoning In Algebra Answer Key
Worksheer Ila Shape & Polarity Electron
Worksheer Ila Shape & Polarity Electron-Group Geometry: Molecular Geometry Carbon dioxide CO2 Lewis Structure Perspective Drawing Around Central Atom Single Bonds: Angles: e-groups = VSEPR Notation: Double Bonds: Triple Bonds: AEN: Lone Pairs: Polar Non Polar Electron-Group Geometry: Molecular Geometry: Boron trifluoride Lewis Structure Perspective Drawing – B – Around Central Atom Angles: Single Bonds: e-groups VSEPR Notation: Double Bonds: AEN Triple Bonds: Polar Non Polar Lone Pairs:
Is Ccl4 Polar Or Nonpolar
Carbon Tetrachloride having its chemical formula as CCl4 is an organic compound. It is a colorless liquid with a sweet smell. It is also toxic to the human body on its direct exposure. We will study the properties of CCl4 in detail. Chemistry students may have doubts about whether CCl4 is polar or nonpolar. So, In this article, I will answer this question and will cover the surrounding topics too.
SO, Is CCl4 polar or nonpolar? CCl4 is nonpolar in nature. Although the four bonds C-Cl are polar because of the difference in electronegativity of Chlorine and Carbon, CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure of the CCl4 molecule.
Carbon Tetrachloride is an IUPAC name given to CCl4 and it exists as a liquid state at room temperature. It produces a sweet odor that can be easily detected at lower levels.
It was discovered by Henri Victor Regnault in the year 1839.
CCl4 molecule has one carbon atoms attached to the four chlorine atom. All four bond C-Cl are similar as well as symmetrical to each other.
It is important to understand that symmetry of a chemical compound is a very important factor in determining the polarity of a compound.
It can be understood because Carbon and Chlorine atom have a different value of electronegativity ie . Making the C-CL bond a polar covalent bond.
Methane gas does also has the same structure as CCl4.
Read Also: What Does Abiotic Mean In Biology
Sketch Out A Skeleton Of The Compounds Atomic Structure
Next up is to figure out the atomic organization of the compound. If the compound is diatomic , then this is easy: the atomic structure will just be the two atoms sitting next to each other in a straight line. In a compound with three or more atoms, things get a bit more complicated. In most compounds with more than three atoms, there tends to be a central atom that shares bonds with multiple atoms.; The central atom tends to be the least electronegative element of the compound.
In our case, carbon is less electronegative than chlorine, so carbon is the central atom.; We can sketch our diagram with a central carbon atom surrounded by 4 chlorine atoms, like this:
Is Ccl4 Polar Or Non
When it comes to polarity of a compound, we must understand the fact that it is directly related to its structure and shape. Molecules having their charges unevenly distributed are said to be polar in nature, whereas, those molecules whose charges are evenly distributed are non-polar. In case of CCl4, the charges are distributed uniformly around the central carbon atom. Due to this symmetry, tetrachloromethane is said to be non-polar, even though it contains polar bonds.
Also Check: Algebra Road Trip Project Answer Key
Lewis Structures: The Basics
Lewis structures;were first introduced;by the American chemist G.N Lewis in 1916. Since then, they have become ubiquitous in high school and college level chemistry courses as an easy way to understand chemical bonding.
Lewis structures;are meant to represent the atomic and electron structure of a chemical compound. Each element of the compound;is represented in the Lewis structure by its chemical symbol, so H for hydrogen, C for carbon, O for oxygen, and so on. The configuration of the elements electron shell is represented by a pattern of dots that surround the chemical symbol.;Shared electron;pairs;are represented as a single line that connects the two bonded elements. Lone pairs of electrons are represented as a pair of lone dots next to a chemical symbol.
How many dots are supposed to be around a symbol;is determined by the elements;valence numberthe number of electrons in its outer shell. Oxygen, for example, has a valence number of 6 because it has 6 electrons in its outer shell. Most elements will seek to fill their outer shell entirely and will bond with other elements until their;valence number is 8, corresponding to a full outer shell of 8 electrons. The tendency for elements in compounds to arrange themselves to have a full valence shell of 8 electrons is called the;. The lone exception to the octet rule is hydrogen. Hydrogen has a full outer shell with only 2 electrons and so will form bonds until it has 2 electrons.
How To Draw Carbon Tetrachloride Lewis Structure
CCl4 lewis structure contains four chlorine atoms and one carbon atom connected with 4 single bonds. The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram.
To draw the stable Carbon tetrachloride lewis structure, we have to represent the valence electron of each atom within a molecule.
Lets see how to do it step by step.
Don’t Miss: What Does I Stand For In Physics
Find The Number Of Lone Pairs Present On The Central Atom Of The Ccl4 Lewis Structure
As per the CCl4 lewis structure, carbon is the central atom that has no lone pair present on it because carbon completes its octet with the help of 4 single bonds.
Or you can determine the lone pair in CCl4 by using the simple formula.
L.P = /2
where L.P. = Lone pair on the central atom
V.E. = valence electron of that central atom
N.A. = Number of atoms attached to that central atom
So, the valence electron of Carbon is 4, and the number of the attached atom to Carbon is also 4.
Put these values in the given formula-
/2
= 0 is the lone pair present on the central atom.
Lewis Dot Structure For Ccl4
The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. The electrons are represented with the help of circular dots. This diagram displays the bonds formed as well as lone pairs of electrons.
Consider the diagram given above. Carbon forms one covalent bond with each chlorine atom, which is electronegative in nature. As we already know, the atomic number of carbon atom is 6, and that of chlorine is 17. Thus, carbon and chlorine have 4 and 7 valence electrons, respectively. This implies that carbon requires 4 electrons and chlorine requires 1 electron to satisfy its octet. The carbon atom shares 1 electron each to form one covalent bond with every chlorine atom to achieve its octet.
Total number of electrons in the compound = Total number of valence electrons of Total number of electrons in the compound = + = = 32
The number of electrons around the carbon atom is 8. The remaining 24 atoms must be distributed in the structure in such a way that all the atoms have 8 electrons in their valence orbital. Considering the four atoms of chlorine, each atom will have 6 electrons around it. The atom of each element present in this compound is stable, in turn, making the compound quite stable.
You May Like: Pre Algebra Order Of Operations Help
Why Ccl4 Is Nonpolar
The CCl4 is nonpolar in nature because of the symmetrical tetrahedral geometrical structure. Although the C-CL bond is polar in nature as Carbon and Chlorine atoms have a difference in their electronegativity.
As a result, the C-Cl bond also has a dipole moment. But due to symmetrical structure, the net dipole moment gets canceled with each other forming CCl4 a nonpolar molecule.
The polarity of a molecule depends upon the various factors discussed below.
Molecules With More Than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. We take in account the geometric distribution of the terminal atoms around each central atom. For the final description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the example provided below:
Butane is C4H10. C-C-C-C is the simplified structural formula where the Hydrogens are implied to have single bonds to Carbon. You can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. Let’s start with the leftmost side. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. That means that we have 4 electron groups. By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens.
Don’t Miss: How To Find Biological Grandparents Uk
Ccl4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether.
Initially, the compound had everyday applications but considering its harm to humans the chemical is now banned from use.
The compound is clear and very stable in nature.
CCl4 is also named carbon chloride, methane tetrachloride, benziform, and more.
The liquid is not soluble in water and is non-combustible.
The boiling point of CCl4 is 76.8 degrees Celcius and its melting point is -23.0 degrees Celcius. CCl4 will release toxic fumes like carbon monoxide. if it is led to decomposition.
If any human inhales CCl4 compound or gets orally exposed to it then they can feel headache, lethargy, weakness, and nausea.
If the compound is consumed or exposed to humans in large or continuous motion then the person or group of people can suffer from kidney or liver damage.
These are some of the basic properties of Carbon tetrachloride.
Now lets move into the study of its structure and other details, starting from its Lewis structure.