H2o Molecular Geometry And Bond Angles
H2O has a tetrahedral arrangement of molecules or an angular geometry. This is mainly because the repulsion from the lone pair combination is more than bond-pair repulsion. Additionally, the existing pairs do not lie in the same plane. One pair is below the plane and the other one is above. This bond geometry is commonly known as a distorted tetrahedron.
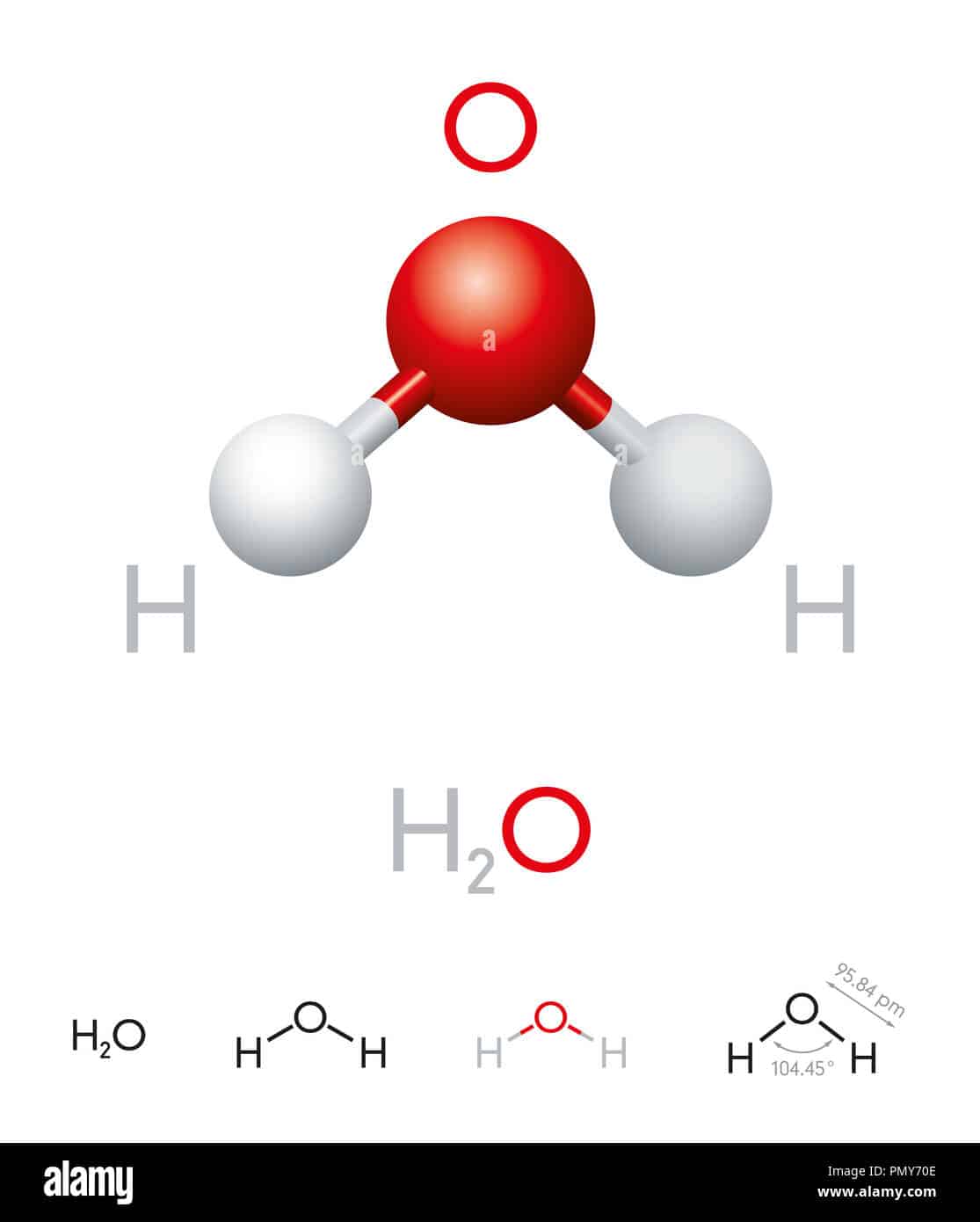
As a result, the angle in a water molecule is 104.5° which again falls short of the true tetrahedral angle of 109°.
What Are The Valence Electrons
The valence electrons are free electrons present in the outermost shell of the atom. The nucleus holds the outer shell weakly as it is farthest in the distance.
Moreover, if the valence electrons are unpaired, they become highly reactive in nature by either accepting or donating electrons to stabilize its outermost shell.
It is interesting to realize that the larger the number of valence electrons, the stronger will be the ability to accept the electrons.
Whereas, the smaller the number of valence electrons, the stronger will be the ability of the atom to donate them.
Understanding The Electronic Geometry Of H2o
The H2O molecule is composed of two hydrogen atoms and one oxygen atom. It forms a bond angle of 104.5°. As a result, it is feasible to determine that it is bent in the form of an H2O molecule.
According to Lewiss structure, a lone pair exists when all of the atoms valence electrons are unpaired.
The H2O molecules Lewis structure reveals two solitary sigma bonds between the O and H. Additionally, and these connections leave two lone pairs of electrons on the oxygen atom, which adds significantly to the H2O molecules tetrahedral bent geometrical configuration.
You May Like: What Is Spread In Math
What Is The Bond Angle For H2o
104.5°The actual bond angle in the water molecule is 104.5°.
Is water a trigonal planar?
Trigonal planar: Molecules with the trigonal planar shape are somewhat triangular and in one plane . Consequently, the bond angles are set at 120°. For example, water , which has an angle of about 105°. A water molecule has two pairs of bonded electrons and two unshared lone pairs.
What is the molecular geometry of H2O enter the molecular geometry of the molecule?
To get the total number of valence electrons for this molecule, we will add up Hydrogen and Oxygen atoms valence electrons.H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles.
| Name of molecule |
|---|
| Bent |
Is there a polar bond in H2O?
Water , like hydrogen fluoride , is a polar covalent molecule. When you look at a diagram of water , you can see that the two hydrogen atoms are not evenly distributed around the oxygen atom.
The Geometrical Structure Of The H2o Molecule
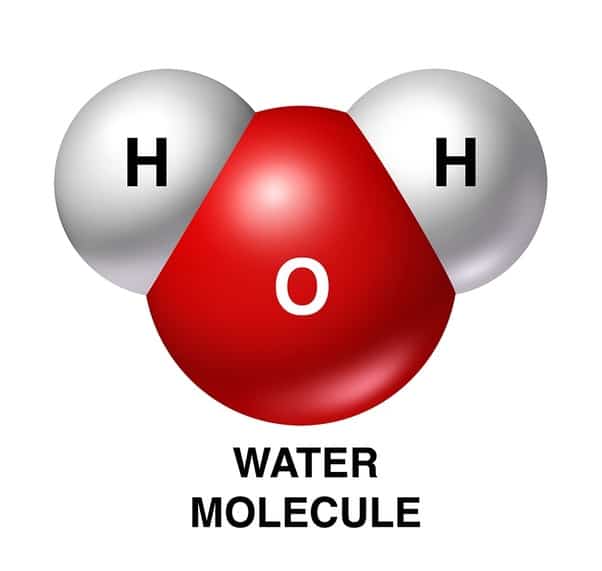
The bond angle among hydrogen-oxygen-hydrogen atoms is 104.5°. From this, it can be understood that the geometrical structure of a single H2O molecule is bent.
It is explained with the help of the Valence Shell Electron Pair Repulsion theory, which says why irrespective of having two pairs of lone electrons on the oxygen atom the bond angle is reduced to 104.5°.
The ideal bond angle for a bent-shaped molecule is 109.5°.
According to the Lewis structure, there exists lone pair when all the valence electrons around the atom are not paired.
Similar is a case of the oxygen atom in the H2O molecule, where two lone pairs exist.
These lone pairs distort the bond angle due to the lone pair-lone pair, which is more than the bond pair-bond pair and lone pair-bond pair repulsion.
When the lone pair increases, the bond angle decreases. As there are two lone pairs on the oxygen atom, it reduces the bond angle to 104.5°.
Read Also: What Does Denominator Mean In Math
How Can We Determine The Molecular And Electron Geometry Of H2o
The molecular geometry deals with the position of the nucleus of the different atoms in the molecule whereas the electron geometry deals with the position of the orbitals. Electron geometry is specific for each atom in the molecule. According to VSEPR theory, valence electrons repel more than bonding electrons. This can be used to determine the geometry as we know that the oxygen atom in H2O has 2 pairs of valence electrons and 2 pairs of bonding electrons. There are therefore 4 electron domains on the oxygen atom which suggests a tetrahedral electron geometry . Because not all electron domains are counted in the molecular geometry, the shape of the molecule H2O is bent .
What Is The Molecular Geometry Of H2o
Water has 4 regions of electron density around the central oxygen atom . These are arranged in a tetrahedral shape. The resulting molecular shape is bent with an H-O-H angle of 104.5°.
What is the molecular geometry of hs2?
H2S molecular geometry is bent. H2S electron geometry is tetrahedral. The total valence electron available for drawing the lewis structure of H2S is 8.
Don’t Miss: How To Calculate Tension In Physics
What Are The Electron Geometry And The Molecular Geometry Of Water
The electronic geometry gives water a tetrahedral shape.The molecular geometry gives water a bent shape.
Explanation:
Electronic geometry takes into account the electron pairs that are not participating in bonding, and the electron cloud density.
Here the 2 bonds of hydrogen count as 2 electron clouds, and the 2 electron pairs count as another 2, giving us a total of 4. With 4 electron regions, the VSEPR electronic geometry is tetrahedral.
Molecular geometry looks at only those electrons that are participating in bonding. So here, only the 2 bonds to H are taken into account.
The shape would not be linear, as in the case with #”CO”_2#
H2o Lewis Structure Molecular Geometry And Hybridization
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound.
It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table.
The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound.
The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs.
The maximum number of dots that can be drawn is eight per atom, as per the octet rule. Moreover, the formation of a bond because of reacting valence electrons are shown with the help of the lines.
The atomic number of a hydrogen atom is one, which makes its electronic configuration 1s1. As the 1s shell can accommodate a maximum of two electrons, there is a dearth of one more electron.
It makes a single hydrogen atom to have one valence electron.
Besides this, in the case of oxygen, its electronic configuration is 1s2 2s2 2p4 where 2p shell can accommodate six electrons.
Read Also: What Does Biological Sister Mean
What Is The Hybridization Of Water
In this section, we will basically understand the formation of water on the basis of hybridization. The central atom here is oxygen which is hybridized. So if we observe the formation of the water molecule there are three 2p orbitals and one 2s orbital. These combine to create the four sp3 hybrid orbitals.
Further, in the process, two-hybrid orbitals form covalent bonds with each hydrogen atom and two hybrid orbitals are occupied by lone pairs.
Waters Molecular Geometry And Bond Angles
The angle between the bonds of hydrogen with oxygen are between 104.5 to 109. It has a V-shaped geometry and is categorised under the tetrahedrons.
Table of Content
Water is the most abundantly found resource, as all the oceans, seas, rivers, and water bodies comprise of water. When we first begin to study, we get to know that the chemical formula of water is H2O. This means that in a single water molecule there are two hydrogen atoms and one oxygen atom. Another name for the water molecule is Dihydrogen Monoxide Like all other chemical compounds, water too has bonds and here we will discuss the geometry and angles that the water compound makes.
The structure is scientifically known as the Lewis Structure and popularly named the Electron-dot Structure which means that it represents the molecule of water diagrammatically. This in turn makes the number of valence electrons or the electrons in the last shell of the atom known. The number of valence electrons makes sure the number of electrons that are free to create bonds and then a compound. The prediction of the geometrical shape of the molecule of water is done on the theory of Valence-Shell Electron Pair Repulsion .
Don’t Miss: Does Doing Math Increase Iq
All About The Electronic Geometry Of H2o
If youre a chemistry buff, then you know that water is one of the most important molecules in the world. Not only is it essential for life as we know it, but its also incredibly complex. In this blog post, were going to focus on one aspect of water molecules: their electronic geometry. Keep reading to learn more about this fascinating topic, what is the electronic geometry of h2o?!
Contents
What Is The Octet Rule
According to the Octet rule, the maximum of valence electrons that an atom can have is eight. Moreover, these eight electrons are drawn only around the symbol of the atom in the Lewis structure.
The oxygen has a dearth of two valence electrons. Whereas, the two hydrogen atoms have a dearth of two valence electrons in total.
The Lewis structure of H2O is drawn in such a manner that the deficiency of each atom is fulfilled.
Also Check: How To Find Biological Grandparents Uk
The Importance Of Electronic Geometry Of H2o In Science And Experiments
The electronic geometry of water is both simple and complex. On the one hand, it is made up of just two atoms of hydrogen and one atom of oxygen. On the other hand, the arrangement of these atoms gives water some remarkable properties.
- For instance, water molecules are able to form strong bonds with each other, and this gives rise to the liquids high surface tension.
- Additionally, the electronic geometry of water enables it to act as a solvent for a variety of substances.
In fact, without water, many chemical reactions would simply not be possible. Therefore, it is clear that the electronic geometryof water plays a vital role in both science and experiments.
As you can see, the electronic geometry of water is a fascinating topic with far-reaching implications.
Why There Is No Lone Pair Around The Outer H Atoms In H2o Lewiss Structure
The electronic configuration of hydrogen is 1s1. Naturally, it is short of a single electron only to achieve its most stable duplet configuration. Therefore, H atoms only form a single bond with the central O atom in the Lewis structure of H2O.
Once that is done, both the H-atoms complete their duplet, and no electrons are required as lone pairs around the outer H-atoms.
Also Check: What Is Cultural Diffusion In Geography
Hybridization Of H2o Molecule
The bond between each oxygen and hydrogen atom in a water molecule is sigma with no pi bonds.
As we know, sigma bonds are the strongest covalent bonds. As a result, there is high stability between the oxygen and the hydrogen atom.
It is the two lone pairs on the oxygen atom which makes all the difference. The hybridization of a water molecule is sp3, where its oxygen has been hybridized.
According to the diagram, it can be analyzed that the single oxygen atom in the water molecule has one 2s orbital and three 2p orbitals. These four altogether leads to the formation of four sp3 hybridized orbitals.
It leads to the formation of the tetrahedral bent geometry, where overall H2O molecule shows 25% characteristics of s and 75% characteristics of the p orbital.
It can further be explained with the help of a molecular orbital diagram of the H2O molecule.
The 2s orbital and three 2p orbitals of the oxygen atom forms four new hybrid orbitals which further bonds by undergoing overlapping with the 1s orbital of the hydrogen atoms.
Molecular Orbital Diagram Of Water
The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound.
Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure.
From the above diagram, it can be seen that the six valence electrons are bonding with the 1s orbital electrons of the hydrogen atom.
The mixing and overlapping are occurring among the atomic orbital of similar energy.
It is taking place in such a manner that the bonding electrons in lower energy are forming antibonding molecular orbitals of higher energy.
The left oxygen electrons do not overlap further due to the scarcity of electrons.
The oxygen atom has its electronegativity higher than hydrogen. Due to this, oxygen has a higher negative charge, whereas hydrogen has a positive charge. It makes oxygen attract nearby electrons and form a bond ultimately.
On the other hand, the hydrogen does not react with nearby molecules as it has already fulfilled its orbital and bonded with oxygen through a sigma bond, which is not easy to break.
It leads to the formation of polarity in an H2O molecule, irrespective of having a net neutral charge.
You can also check an interesting article written about the polarity in water.
Don’t Miss: What Is Excretion In Biology
Molecules With More Than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. We take in account the geometric distribution of the terminal atoms around each central atom. For the final description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the example provided below:
Butane is C4H10. C-C-C-C is the simplified structural formula where the Hydrogens are implied to have single bonds to Carbon. You can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. Let’s start with the leftmost side. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. That means that we have 4 electron groups. By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens.
Why H2o Has A Bent Shape And Not Linear
There are 2 lone pairs present on the central O atom in the H2O molecule. Lone pair-lone pair repulsions and lone pair-bond pair repulsions exist in the molecule. Therefore, the molecule cannot attain a linear shape.
Rather, it adopts an asymmetric bent shape to minimize the repulsive effect. The O-H bonds tilt inwards and the H-O-H bond angle decreases.
Don’t Miss: What Math Class Do 11th Graders Take
Why Is The Molecular Geometry Of Water H2o Bent And Not Linear
The two hydrogen atoms and the two lone electron pairs are as far apart as possible at nearly 109obond angle. The water molecule is bent molecular geometry because the lone electron pairs, although still exerting influence on the shape, are invisible when looking at molecular geometry.
What is the shape of H2O?
Is H2o Bent Or Linear
The water molecule is bent molecular geometry because the lone electron pairs, although still exerting influence on the shape, are invisible when looking at molecular geometry.
How many lone pairs does Hono have?
2 free lone pairsThe remaining unshared valence electrons constitute the lone pairs. There will be double bond nitrogen and one oxygen atom to minimize lone pairs. Thus, the structure of HONO is: Hence, it is quite clear from the above structure of the HONO molecule that, on one oxygen atom, there are 2 free lone pairs.
You May Like: How Did Geography Discourage Greek Unity
S Used To Find The Shape Of The Molecule
To sum up there are four simple steps to apply the VSEPR theory.
Why Is Electronic Geometry Important
Knowing the electronic geometry of a molecule is important because it helps us understand its reactivity.
- For example, water molecules with tetrahedral geometry are much less reactive than those with linear geometry because the former has a higher bond angle . This means that it takes more energy to break apart water molecules with tetrahedral geometry.
- In addition, electronic geometry can also help us predict things like melting and boiling points. For instance, water molecules with linear geometry have lower melting and boiling points than those with tetrahedral geometry because linear molecules can slide past one another more easily.
Read Also: What Is Mental Set In Psychology