Pka And Buffer Capacity
In addition to using pKa to gauge the strength of an acid, it may be used to select buffers. This is possible because of the relationship between pKa and pH:
pH = pKa+ log10
Where the square brackets are used to indicate the concentrations of the acid and its conjugate base.
The equation may be rewritten as:
Ka/ = /
This shows that pKa and pH are equal when half of the acid has dissociated. The buffering capacity of a species or its ability to maintain pH of a solution is highest when the pKa and pH values are close. So, when selecting a buffer, the best choice is the one that has a pKa value close to the target pH of the chemical solution.
Pi Bonds And P Orbitals
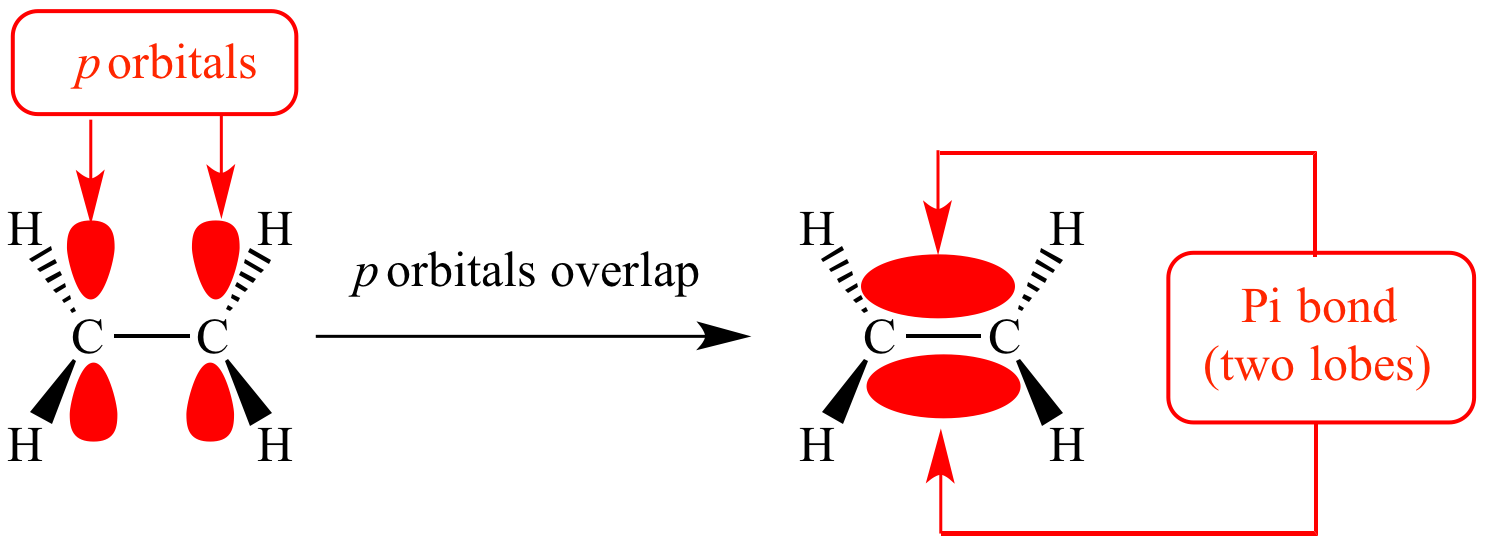
The reason that pi bonds form above and below the bonding axis but not along it is because they form usually from overlapping orbitals such as p orbitals on the bonded atoms. These orbitals do not have an electron density at the nucleus. As a result, the electrons making up the pi bonds that form from the overlapping p orbitals will always cluster in a region that is not directly adjacent to the nucleus. Pi bonds can also form between other atomic orbitals, such as d orbitals which have features in common with p orbitals.
The Definitive Guide To Ph Pka And Pi
We all rely on pH, pKa, and pI for a vast amount of our research. But what is pH? How does it relate to pKa and pI?
These numbers fall into that category: things I learned eons ago, and now Im too embarrassed to ask what they are.
Fortunately, reading an article on the internet is discreet.
And here at Bitesize Bio, we dont judge.
Need to quickly re-learn what these parameters are so that you can confidently train an inductee? Been handed an urgent lab task that requires you to fully understand these numbers to do a thorough job of it?
Stick around, be anonymous, and learn the answers to what is pH? what is pKa? and what is pI without ignominy.
Read Also: How To Calculate Net Force
What Are Conjugated Compounds In Organic Chemistry
The delocalization of electrons in a molecule is called conjugation in organic chemistry. This delocalisation process of electrons leads to the shortenings or elongations of chemical bonds, but at the same time it causes changes in the chemical properties in conjugated molecules as compared to the non-conjugated ones. For example, conjugated molecules absorb light at longer wavelengths.
Conjugated compounds are those compounds at which pi- bonds are present in alternating positions of compounds it can also be defined as compounds having pi-bonds and positive charge or negative charge at alternative positions. Alternative position is always called a conjugation position.
Chemical Bonding In A Conjugated System
- Formation of the conjugated system is only possible when there are alternating single and double bonds in which each atom has the ability to supply a p-orbital perpendicular to the plane of the molecule.
- Along with that, conjugated system can also be formed if an atom has p-orbital in a continuous atom chain. Example of such conditioning is Furan (it is a five membered ring having alternative double bond
- One of the most common models which is used for treatment of conjugated molecules is composite valence bond or Huckel Molecular Orbital Theory treatment.
- Under the treatment procedure, the framework of the molecule is separated from the system of the molecule. Along with that, we can also treat pi- bonding by using the approach of delocalization of electrons.
You May Like: How Did Geography Affect The Development Of The Greek City-states
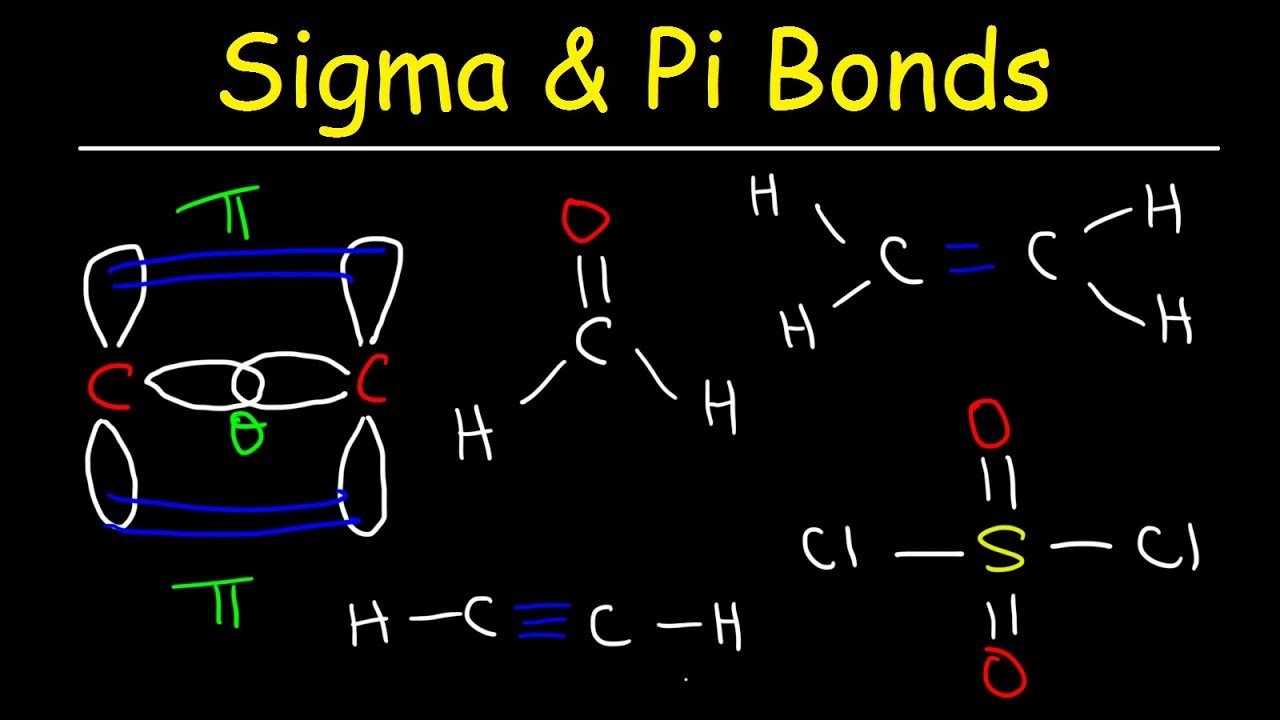
Differences Between Sigma Bonds And Pi Bonds
Despite their similarities, there are important differences.
- Electrons making up sigma bonds will be distributed in the space along the axis connecting the joined nuclei whereas electrons within pi bonds will be distributed above and below the axis but not along it.
- Sigma bonds are the first bonds to form between atoms within molecules whereas pi bonds are the second.
- Sigma bonds are often formed by the combination of s orbitals in different atoms whereas pi bonds are formed from the combination of p and similar orbitals in different atoms.
- Additionally, the orientation of overlapping orbitals that form pi bonds will be perpendicular to that of overlapping orbitals that form sigma bonds.
N To Sigma Star Transition:
Transition occur in saturate hydrocarbons/ molecules containing hetero atoms with lone pair of electrons. These transition involve less energy than sigma to sigma star transition. This shows absorption between 150-200nm. For example, 1- Methyl alcohol has an n-sigma star absorption band at 183nm 2- CH3 NH3
Recommended Reading: Geometry Homework Practice Workbook Answers
Antibonding Pi Molecular Orbitals Result From The Destructive Side
The second molecular orbital is described by destructive overlap where p orbitals of mismatched phase join together to form another pi molecular orbital.
Note that this results in a node between the atoms an area where there is a change in phase and thus no orbital overlap . This corresponds to the antibonding situation we described earlier, where the two nuclei are held closely together in space but lack the attractive interactions of electrons between them. Hence it is higher in energy.
Energy Diagram Including Bonding Non
So, like relationships, in chemical bonding weve seen three situations of differing energy.
- nonbonding the energy of the atom in the absence of any bonding.
- bonding the energy in the happy situation where the two electrons are between the atoms, which is more stable: attraction > repulsion
- anti bonding the energy in the unhappy situation where the electrons are held away from the atoms: repulsion > attraction
For bonding between two identical atoms, we can roughly sketch out these three levels as follows.
Note that we havent populated the levels with electrons yet. That comes next.
Don’t Miss: Test Form 2b Answers Chapter 7
Characteristics Of A Sigma Bond
- The sigma bond is formed by head-on-head overlapping of hybrid orbitals.
- The sigma bond is strong and stable.
- The sigma bond formation is the first step taking place when two atoms or molecules interact with one another.
- The sigma bond is usually denoted by the symbol .
- All alkanes, alkenes, and alkynes exhibit sigma bond formation.
Determination Of The Isoelectric Points Of Proteins And Peptides By Cief And Ce
pI values of peptides or proteins can be determined also by CE . In this case, the electrophoretic mobilities of the peptides or proteins are measured at different pH and the obtained values are then plotted as function of pH. From these plots, the isoelectric points of peptides or proteins are determined as pH values at which the mobility curves intersect the zero mobility line. With both CIEF and CE methods, it is important to perform pI measurements at a constant temperature.
Adamina Vocero-Akbani, … Steven F. Dowdy, in, 2000
You May Like: Books Never Written Pre Algebra With Pizzazz
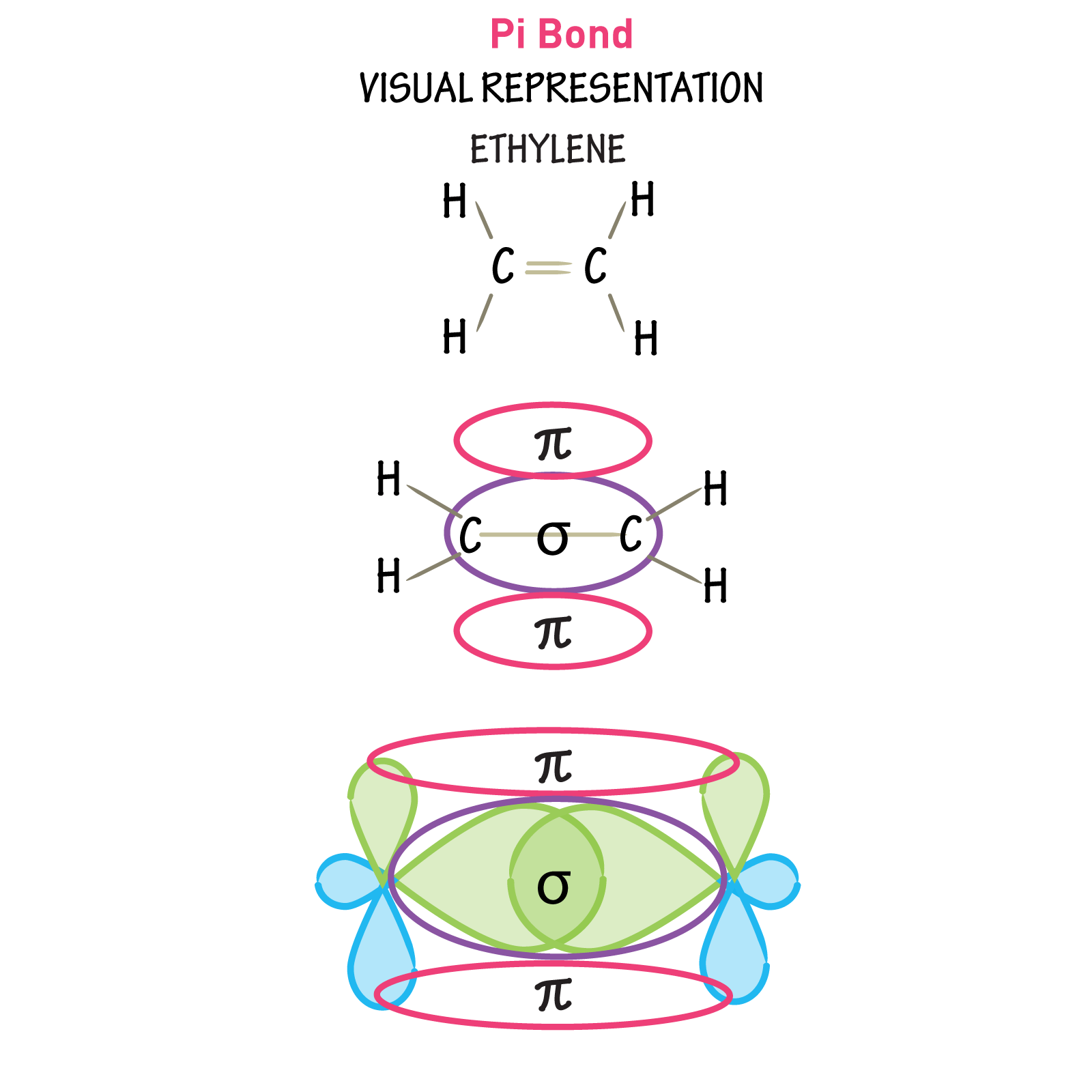
What Are Pi Bonds
Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. Pi bonds are often written as bonds, where the Greek letter refers to the similar symmetry of the pi bond and the p orbital.
The two orbitals which are bonded share the same nodal plane at which the electron density is zero. This plane passes through the nuclei of the two bonded atoms and is also the nodal plane for the molecular orbital corresponding to the pi bond.
An illustration depicting the overlapping of two different p orbitals in order to form a bond is given below.
Commonly, pi bonding involves p orbitals. However, d orbitals also have the ability to participate in bonding and these types of bonds involving d orbitals can be found in the multiple bonds formed between two metals.
A Refresher On The Acid Dissociation Constant Ka
We can take any acid and label it HA where H is the proton associated with the conjugate base, A.
In solution, HA will dissociate into a free proton, H+, and free conjugate base, A. Using the squared bracket notation mentioned already, we can write:
HA -> H+ + A
And given that stoichiometry must be obeyed, using the squared bracket notation described already, we can write:
When the equilibrium is achieved, the concentrations of products and reactants are no longer changing. So the ratio of the products to reactants is also not changing.
The product:reactant ratio is constant. This is the acid dissociation constant, Ka.
Ka = /
The larger the value of Ka, the higher the product:reactant ratio.
Thats to say, the higher the value of Ka, the more free protons there are in the solution.
This, by definition, means the larger the value of Ka, the stronger acid we are dealing with.
Rememberstronger acids have larger Ka values than weaker acids.
You May Like: Homework 5 Angle Addition Postulate Answer Key
Orbitals And Sigma Bonds
Orbitals are regions around atoms associated with certain energy levels. Electrons in orbitals farther from the nucleus will have more energy than electrons in orbitals closer to the nucleus. When the orbitals of one atom overlap with the orbitals from another atom, they form molecular orbitals that allow for molecular bonds which, of course, allow for molecules.
Sigma bonds are the first type of bond that will form between atoms. Within a sigma bond, the electron probability clouds will be along the axis connecting the nuclei of the bonded atoms. Sigma bonds will typically form when s orbitals from different atoms overlap to create a bond. They will always form along the axis between the two nuclei because the s orbital is arranged in something like a sphere around the nucleus.
Pi Day Challenge: Can You Solve These Nasa Math Problems
NASAs Pi Day Challenge includes the release of four science and engineering questions related to NASA missions. Answers will be made public on March 15. Credit: NASA-JPL/Caltech
To celebrate Pi Day, NASAs Jet Propulsion Laboratory is serving up a series of science and engineering questions related to some of the agencys Earth and space missions.
Its deliciously reliable, like cherry pie: Divide the circumference of any circle in the universe by its diameter, and you will always get the same number, pi, aka the Greek letter p. In fact, NASA relies on pi for all sorts of applications.
Though it has an infinite number of decimals, the mathematical constant is usually abbreviated to 3.14, which is why Pi Day is celebrated on March 14. To mark the occasion this year, the STEM engagement office at NASAs Jet Propulsion Laboratory in Southern California has released a quartet of illustrated science and engineering questions related to NASA missions: the upcoming Lunar Flashlight and SWOT missions, along with InSight and TESS .
Answers to all four challenge questions will be made public on March 15.
Need another serving? Previous years challenge questions are online as well.
Now in its ninth year, the NASA Pi Day Challenge is accompanied by other pi-related resources for educators, K-12 students and parents, including lessons and teachable moments, articles, downloadable posters, and web/mobile backgrounds.
Read Also: Beth Tomas
Chemical Reaction Taking Place In The Solution:
Chemical reaction of several ions can take place in the solution without the analyst knowledge. If any of them change the concentration of absorbing specie an appears deviation from beer law is observe/ result. The concentration of absorbing specie is decrease as a result of chemical reaction a negative deviation is observe. A positive deviation from beer law occurs when concentration of an absorbing specie is increases by chemical reaction. Among these types of chemical reaction which can lead to deviation from beer law are acid-base reaction polymerization reaction.
Pi Bonding In Multiple Bonds
The following multiple bonds can be broken down into sigma, pi, and delta bonds:
- Generally, double bonds consist of a single sigma bond and a single pi bond. An example of such a bond can be seen in ethylene .
- Typically, triple bonds consist of a single sigma bond and two bonds which are placed in planes that are perpendicular to each other and contain the bond axis.
- Quadruple bonds are very rare bonds which can only be found in the bonds between two transition metal atoms. It can be broken down into one sigma, two pi, and one delta bond.
The combination of pi and sigma bonds in multiple bonds are always stronger than a single sigma bond. The reduction in bond lengths in multiple bonds points towards this statement. This contraction in the bond length can be observed in the length of the carbon-carbon bond in ethane, ethylene, and acetylene, which are equal to 153.51 pm, 133.9 pm, and 120.3 pm respectively.
Therefore, it can be understood that multiple bonds shorten the total bond length and strengthen the overall bond between the two atoms. To learn more about sigma and pi bonds, register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: Is Physics Harder Than Chemistry
What Is Bipy In Chemistry
4.1/5Bipyridinebipyridine
Thereof, is Bpy neutral?
Bipyridine Is A Neutral Chelating Ligand Tha
Additionally, what shape is Fe BIPY 3 2+? 2+ is octahedral, containing a central low spin d6 Ru ion and three bidentate bpy ligands. The complex is chiral, with D3 symmetry. It has been resolved into its enantiomers, which are kinetically stable.
Likewise, is BIPY a pi acceptor?
cyanogen, NC-CN). Charge donation into such systems results in stabilization, therefore bipyridine is found among the pi–acceptor ligands.
Is pyridine a neutral ligand?
A ligand can be an anion or a neutral molecule that donates an electron pair to the complex . Rule 2: Neutral Ligands.
Characteristics Of A Pi Bond
- The pi bond is formed by the overlapping of hybrid orbitals above and below the bonding axis.
- The pi bond is weak and less stable.
- The pi bond formation takes place after the formation of the sigma bond.
- The pi bond is usually denoted by the symbol .
- Alkanes do not exhibit pi bond formation. Unsaturated compounds such as alkenes and alkynes exhibit pi bond formation.
You May Like: Punchline Bridge To Algebra 2nd Edition Answer Key
Where Does Pi Come From
By definition, pi is the ratio of the circumference of a circle to its diameter. In other words, pi equals the circumference divided by the diameter . Conversely, the circumference of a circle is equal to pi times the diameter . No matter how large or small a circle is, pi will always work out to be the same number. Pi is the 16th letter of the Greek alphabet and is used to represent the widely known mathematical constant.
The Full Relationship Energy Diagram
The romantic view of love is that if the potential energy well is deep enough, you have Happily Ever After, lovers destined never to part.
Of course, thats partly a reflection of the fact that romantic comedies cut out right after the wedding scene. They dont check in six months later when Prince Charming has turned into Mr. Never Takes His Kleenex Out of His Pants Before Putting Them In The Dryer and Snow White has morphed into Mrs. Keeps Leaving Her Disgusting Jammy Knife On The Kitchen Counter.
The truth is that even happy relationships possess latent unhappiness. Strain them with enough force, and instability may result.
And this diagram is just for two people!
We dont need to make drawings of the destabilization that can occur when a third person enters the relationship.
Don’t Miss: Examples Of Movement In Geography
Isoelectric Point And Point Of Zero Charge
The isoelectric point and the point of zero charge reflect the response of a surface to an electrolyte, typically water. Through protonation and deprotonation reactions, as a result of the amphoteric nature of water, positive or negative charges can be generated on the surface. Because acidic and basic sites can coexist on surfaces, both types of charge may be formed simultaneously in different locations. IEP and PZC provide information about the type of charge that prevails, which is determined by number and strength of all acidic and basic sites present. IEP and PZC are well suited to describe whether a surface is overall more acidic or more basic.
More specifically, the IEP characterizes the conditions, that is, the concentration of charge-determining ions, at which in electrokinetic measurements no charge is measured, for example, when the electrophoretic mobility of colloidal particles in a dispersion is zero. The IEP reflects the situation when the -potential of the surface is zero. The PZC charge represents the situation when the surface charge density is zero.124 PZC and IEP become equal under pristine conditions .174 IEP and PCZ are typically specified through the pH.
Table 2. Isoelectric points of common oxides
| Compound |
|---|
ALOIS G. PÜNTENER, in, 2000
Deviation From Beers Law:
A plot of absorbance as a function of concentration for a substance which obey beer law results in a straight line. If the observe absorbance for a particular solution is greater than that which would have been expect. Meanwhile, The solution has positive deviation from beer law. Therefore, If the observe absorbance is less than that which would have been expect the solution exhibit negative deviation from beer law.
You May Like: Does Kamala Harris Have Children Of Her Own
Relationship Energy Diagrams: Bonding And Antibonding
A lot of people say theyre happy being single, and I believe that many likely are. But in the back of their mind of many single people is the thought that if they just found the right person, they might be even happier or less unhappy, which is a crappy way to look at it psychologically but necessary if you wish to draw a diagram where a happy couple is occupying a potential energy well, below.
The decrease in unhappiness brought about by bonding can be quantified as the bond energy.
Of course, it can take a lot of random collisions before two single people fall into the potential energy well that categorizes a happy relationship.
Worse, two people can suddenly find themselves in a relationship where they were initially attracted to each other, but upon being in close proximity realize that they were actually happier being single after all.
That gives you a situation like the one below which you can think of as antibonding an unhappy, unstable couple, destined to separate back into its individual components.