How Do You Read A Chemistry Notation
Each element is represented by its atomic symbol in the Periodic Table e.g. H for hydrogen, Ca for calcium. If more than one atom of a particular element is present, then its indicated by a number in subscript after the atomic symbol for example, H2O means there are 2 atoms of hydrogen and one of oxygen.
What Do State Symbols Mean
State symbols are written after each formula in chemical equations to show which physical state each substance is in. Brackets are used and they are not usually subscripted although you may come across them written in this way. Aqueous should remind you of the word aqua and means the substance is dissolved in water.
What Is The Difference Of Aqueous And Liquid
The main and basic difference between an aqueous solution and liquids is that a liquid is a state of matter which has some typical characteristics which distinguishes it from other states of matter, i.e., solids and gases whereas an aqueous solution is a solution where the solvent is water, which is a liquid, and some
Don’t Miss: What Grade Is Biology Taught
Difference Between Liquid And Aqueous
Categorized under Science | Difference Between Liquid and Aqueous
Liquid vs Aqueous
A liquid is a state of matter. There being three states of matter, namely, solid, liquid, and gas. They all have their particular features and properties. By aqueous, we actually mean a solution where the solvent is water and some compound is dissolved in it.
LiquidsLiquidity is a state of matter. It has some typical characteristics which distinguish it from solids and gases. The first feature of liquid is that it can flow. Pour a glass of water at a slanting surface, and one can see it flowing from the higher to the lower surface. The second main feature is that it takes the shape of a container. When a liquid is poured into different shapes of containers, they take the shape of each container. When sealed in a container, they apply pressure evenly at all surfaces. The third most distinctive characteristic of liquids is the surface tension. The best example of surface tension is boiling milk in a container. Once it is boiled, it reaches the top and fluffs up like a fuzzy ball but does not immediately flow out. This characteristics leads to the phenomena called wetting.
Summary:
Example Aqueous Solution Chemistry Problems
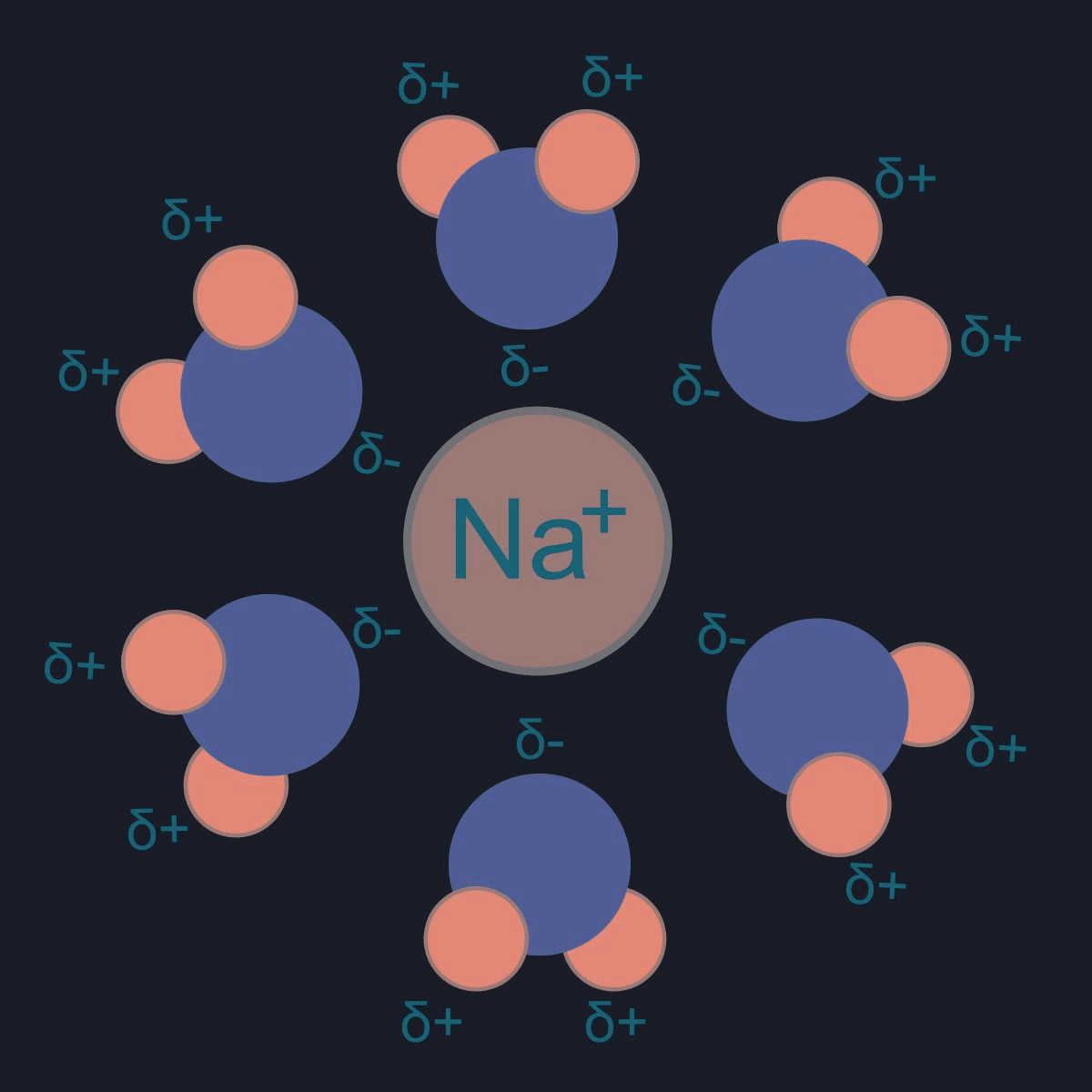
Students encounter a few different types of chemistry problems concerning aqueous solutions. These mainly concern questions of solubility and colligative properties.
Example: Which solute forms an aqueous solution?
- silver hydroxide
Technically, this is not a great question because allionic compounds form aqueous solutions, even if they are very poorly soluble. This is because ionic compounds, like water, are polar molecules. But, the point of a question like this is getting a student to understand solubility rules. Based on these rules, only sodium nitrate is highly soluble in water. Most carbonates, hydroxides, and sulfides are insoluble and these particular compounds are not exceptions to the rules.
Other common questions concern colligative properties. Colligative properties, like freezing point depression and boiling point elevation, depend on the number of particles dissolved in water. The more a compound dissociates into ions or the greater its concentration, the higher it raises boiling point or lowers freezing point.
Example: Which aqueous solution has the lowest freezing point?
- 0.1 molal urea solution
- 0.1 molal sucrose solution
- 0.1 molal sodium chloride solution
- 0.1 molal calcium chloride solution
Example: Which aqueous solution has the highest boiling point?
Also Check: What Does S Stand For In Chemistry
What Is S And Aq In Chemistry
Often chemical equations are written showing the state that each substance is in. The sign means that the compound is a solid. The sign means the substance is a liquid. The sign stands for aqueous in water and means the compound is dissolved in water. Finally, the sign means that the compound is a gas.
What Does Aq Mean In Chemistry
In chemistry, chemical equations are characterized by symbols representing the reactants and products of a reaction.
Typically, the state of the reactants and products is mentioned. A reactions four common states are aqueous, solid, liquid, and gas. In a chemical reaction, aq is a state symbol for aqueous.
The state indicates that the product is dissolved in water.
Contents
Read Also: How To Make Math Symbols With Keyboard
Example: Is Nacl Aqueous Or Solid
Sodium chloride , common salt, can be either a solid or aqueous. When dissolved in water, NaCl becomes an aqueous solution. The salt dissolves into water and dissociates into its constituent ions Na+ and Cl-. The mixture is a neural aqueous solution with a pH of 7.
NaCl naturally exists as a white solid having a definite shape and volume. Its positive and negative ions are held strongly together by electrostatic charges making it have a high melting point. As such, NaCl exists as a solid at room temperature.
Properties Of Aqueous Solutions
Aqueous solutions often conduct electricity. Solutions that contain strong electrolytes tend to be good electrical conductors , while solutions that contain weak electrolytes tend to be poor conductors . The reason is that strong electrolytes completely dissociate into ions in water, while weak electrolytes incompletely dissociate.
When chemical reactions occur between species in an aqueous solution, the reactions are usually double displacement reactions. In this type of reaction, the cation from one reactant takes the place for the cation in the other reactant, typically forming an ionic bond. Another way to think of it is that the reactant ions “switch partners”.
Reactions in aqueous solution may result in products that are soluble in water or they may produce a precipitate. A precipitate is a compound with a low solubility that often falls out of solution as a solid.
The terms acid, base, and pH only apply to aqueous solutions. For example, you can measure the pH of lemon juice or vinegar and they are weak acids, but you can’t obtain any meaningful information from testing vegetable oil with pH paper.
You May Like: Why Is It Important To Understand Geography
Is Co2 A Solid Liquid Gas Or Aqueous
Carbon dioxide, CO2, is usually a gas. It is exhaled by animals and humans and used by plants to produce oxygen. In solid form it is dry ice. Carbon dioxide is a chemical compound that consists of two oxygen atoms and one carbon atom.
Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. After completing his doctoral studies, he decided to start “ScienceOxygen” as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. He also shares personal stories and insights from his own journey as a scientist and researcher.
What Is Mean By Inorganic Chemistry And With Example
Inorganic chemistry is the study of the behaviour of compounds along with their properties, their physical and chemical characteristics. The elements of the periodic table except for carbon and hydrogen are in the lists of inorganic compounds. Many of the elements very important like titanium, iron, nickel and copper.
Read Also: What Is Ima In Physics
Why Is An Aqueous Solution Important
In an aqueous solution where water is the solvent, the solute to be dissolved by the water has fewer particles in it, making the particles move in random motion. Pure water has a low concentration of ions and therefore does not conduct electricity. When a solute dissociates in water and forms an electrolyte, then the solution is a good conductor of electricity.
Solutes that dissociate in water and forms ions are electrolytes. Strong acids and bases in an aqueous solution form a strong electrolyte, which can dissolve completely as a soluble item. Weak electrolytes do not completely dissociate and are usually weak acids and bases. Since strong electrolytes supply ions to the solution, strong electrolytes create aqueous solutions that are more conductive of electricity.
What Is An Aqueous Solution In Chemistry

The aqueous solution containing water and at least one other item is indicated by the symbol after the substance. For example, salt water is a solution indicated by NaCl. Whereas, the components of salt in an aqueous solution are indicated by Na + Cl .
Water only dissolves hydrophilic items including acids, bases and salts. The aqueous solution of these items mixes together with the water completely. Hydrophobic items don’t dissolve very well in water, such as oils and fats.
When you dissolve electrolytes in water, the ions allow the solution to be conductive of electricity. Sugar is a nonelectrolyte and dissolves in water, but at the molecular level it stays intact so the solution is not conductive.
You May Like: Is There Math On The Real Estate Exam
Aqueous Solutions In Geochemistry
In geochemistry, by far the most important solvent is water. In general, geochemists divide natural waters into three types: freshwaters , seawater, and brines . Natural waters also may be intermediates between these types, formed by mixing or by evaporation . The typical chemical characteristics of natural waters are shown in Table 1.
Aqueous Solutions, Table 1 Chemical characteristics of natural waters. Data from several sources (Baas Becking…
This is a preview of subscription content, access via your institution.
What Is Aqueous And Non Aqueous Solution
1. Aqueous solution: The solution in which water acts as a solvent is called an aqueous solution. . 2. Non-aqueous solution: The solution in which any liquid other than water acts as a solvent is called non-aqueous solution.
Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. After completing his doctoral studies, he decided to start “ScienceOxygen” as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. He also shares personal stories and insights from his own journey as a scientist and researcher.
Don’t Miss: What Is The Difference Between Growth And Development In Biology
What Is An Aqueous Solution Definition And Examples
An aqueous solution is a chemical solution in which the solvent is water. The solutes are dissolved molecules and ions that are surrounded by water molecules. An aqueous solution is shown by writing after a chemical formula. For example, an aqueous solution of salt in water is NaCl or Na+ + Cl-. In contrast, a solution in which the solvent is not water is called a non-aqueous solution.
What Is An Aqueous Solution
What Is an Aqueous Solution in Chemistry?
What is an aqueous solution in chemistry? Well, to start, its the 4th state of matter. To a chemist, any substance is found in one of the 4 states of matter. You already know about 3 of them: solid, liquid, and gas. And, in chemistry, an aqueous solution is the 4th state of matter. So, you might say that aqueous solutions matter a lot, as they are literally an entire state, or configuration, of this stuff we call matter.
First thing first, I know there are other states of matter. To your physics teacher, the 4th state of matter would be called plasma. To your chemistry teacher, the 4th state of matter would be called an aqueous solution. A child playing with corn starch might think that oobleck is the 4th state of matter. Oobleck definitely would win the contest if it were based on how fun the name was to say. Yet, at least in chemistry, the 4th state of matter is an aqueous solution. It turns out that an aqueous solution is an extremely practical concept, perhaps unlike plasma and oobleck, which we dont really encounter so much in our everyday lives.
What Is an Aqueous Solution: The Simple Answer
So what is an aqueous solution? It simply means dissolved. Yes, the everyday concept of dissolving something. Thats it. The aqueous solution definition means simply that something has been dissolved in water. The aqueous symbol is .
Aqueous solution definition: something is dissolved in water
Aqueous symbol:
Don’t Miss: Do You Need Biology For Dentistry
Reactions With Aqueous Solution
Many reactions in chemistry and all biological reactions take place in water. Also, we can say that these reactions take place in aqueous solution.
As we know that water has many unique properties and is available plentiful on Earth. Due to this reaction in aqueous solutions occur frequently.
Water molecules are containing two hydrogen atoms bonded to a single oxygen atom. Many substances can dissolve in water and hence give an aqueous solution.
An example of an aqueous solution is sodium chloride i.e. common salt dissolved in water. Three different types of reactions with aqueous solutions are as follows:
1. Precipitation Reactions
These reactions take place when two aqueous reactants, one solid and one liquid, react to form an insoluble product which is called a precipitate.
For example when lead nitrate mixes with potassium iodide as shown in the following chemical reaction:
Pb2 + 2KI => PbI2 + 2KNO3
Lead iodide which produces here is not soluble product and hence is the precipitate.
2. Acid-base Reaction
As we know acid contains positive hydrogen ions. On the other hand, a base is a substance that accepts hydrogen ions and produces negative hydroxyl ions in water.
Due to acid and base reaction, a neutralization reaction occurs.
For example, a neutralization reaction occurs when HCl acid combines with NaOH to produce water and sodium chloride. The chemical equation is:
HCl + NaOH => H2O + NaCl
3. Oxidation-Reduction Reactions
2Na + Cl2 => 2NaCl
Pb2+ + 2I PbI2
Aqueous Solution Definition In Chemistry
WLADIMIR BULGAR / Getty Images
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
An aqueous solution is any solution in which water is the solvent. In a chemical equation, the symbol follows a species name to indicate that it is in aqueous solution. For example, dissolving salt in water has the chemical reaction:
NaCl Na+ + Cl-
Although water is often called the universal solvent, it dissolves only substances that are hydrophilic in nature. Examples of hydrophilic molecules include acids, bases, and many salts. Substances that are hydrophobic do not dissolve well in water and tend not to form aqueous solutions. Examples include many organic molecules, including fats and oils.
When electrolytessuch as NaCl and KCldissolve in water, the ions allow the solution to conduct electricity. Nonelectrolytes like sugar also dissolve in water, but the molecule remains intact and the solution is not conductive.
You May Like: What Does Aromatic Mean In Chemistry
What Is A Chemical Equation
nomenclature
A chemical equation is an expression of a chemical process. For example:
In this equation, AgNO3 is mixed with NaCl. The equation shows that the reactants react through some process to form the products . Since they undergo a chemical process, they are changed fundamentally.
Often chemical equations are written showing the state that each substance is in. The sign means that the compound is a solid. The sign means the substance is a liquid. The sign stands for aqueous in water and means the compound is dissolved in water. Finally, the sign means that the compound is a gas.
Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of molecules, or the relative number of moles . If no coefficient is shown, a one is assumed.
On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, show what conditions need to be present for a reaction to occur. For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe2O3, a temperature of 1000 degrees C, and a pressure of 500 atmospheres for this reaction to occur.
The graphic below works to capture most of the concepts described above:
Example: Is H2o Liquid Or Aqueous

Water can be both a liquid and aqueous solution. Naturally, pure water exists as a liquid, exiting all properties of this state of matter.
Water is a fluid that takes the shape of the container its in. Also, water is incompressible and has surface tension. These four properties make pure water a liquid.
However, water becomes part of an aqueous solution when its used as a solvent for a solute. For example, in a solution of water and NaCl, the salt is the solute, and water acts as the solvent.
The mixture is labeled as an aqueous solution.
Don’t Miss: How To Cheat In Math
Examples Of Aqueous Solubility In A Sentence
-
Prediction of Aqueous Solubility of Organic Chemicals Based on Molecular Structure.
-
Aqueous Solubility for Propellants The dissolution of explosives and propellants has historically been studied at the laboratory scale with soil column tests , batch studies and neat or reagent-grade compounds .
-
Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices.
-
Increase in Aqueous Solubility, Stability and in vitro Permeability of anandamide by Hydroxypropyl–cyclodextrin.
-
Improving the Aqueous Solubility of Triclosan by Solubilisation, Complexation, and in Situ Salt Formation.
-
Sun, High Aqueous Solubility of Functionalized Single-Walled Carbon Nanotubes, Langmuir, 2004, 20, 4777.
-
Lynch, J.C., et al., Effects of pH and Temperature on the Aqueous Solubility and Dissolution Rate of 2,4,6-Trinitrotoluene , Hexahydro-1,3,5-trinitro-1,3,5-triazine , and Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine .
-
Aqueous Solubility and Dissolution Rates for Energetics With the exception of NG, the major energetic compounds used by the DoD as propellants are solids at ambient temperature and are deposited on ranges as particles of solid material .
-
ASTM Standard E 1148-87 “Standard Test Method for Measurements of Aqueous Solubility“, approved April 3, 1987 NTIS.
-
Aqueous Solubility Limits of Sodium Salts of S at Different Solution Temperatures .