Organic Vs Inorganic Chemistry
When the term chemistry is mentioned, many of us imagine these laboratories and the interactions in which materials are mixed and other materials are produced, but the chemistry is more profound.
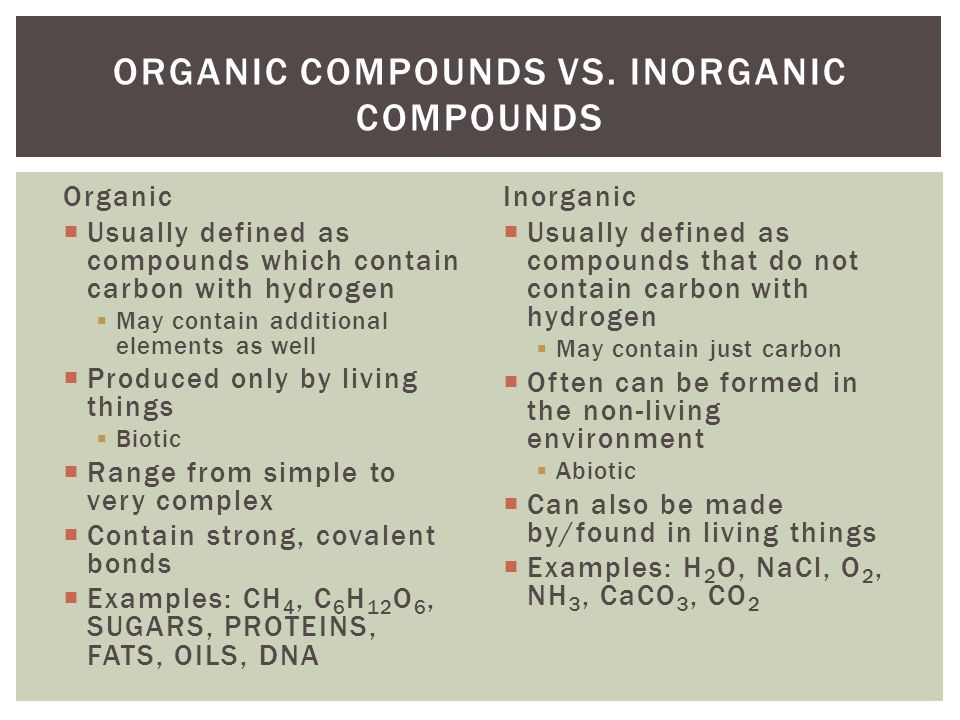
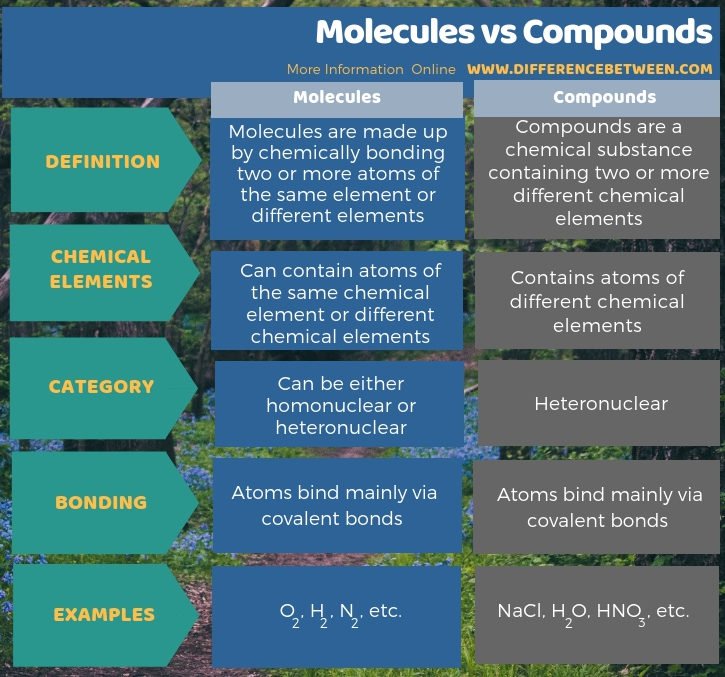
The major difference between organic and inorganic chemistry is that organic chemistry is the study of the structure, composition, properties, reactions, and preparation of carbon-containing compounds including hydrocarbons while Inorganic chemistry;deals with the synthesis and behavior of;inorganic;and organometallic compounds.
What Are Organic Compounds
These are any chemical substances that contain carbon. Numerous organic substances are identified because of the capacity of the carbon to catenate . The study referred to as organic chemistry includes the evaluation of the structures and reactions of organic compounds.
While organic compounds make up just a tiny percentage of the Earths surface, they are of essential significance because organic compounds are the source of all known life.
Living organisms integrate inorganic carbon compounds into organic compounds through a channel of mechanisms starting with the processing of carbon dioxide and supply of hydrogens such as water into simple sugars and other organic molecules, utilizing light or other energy sources.
Characterization Of Inorganic Compounds
Because of the diverse range of elements and the correspondingly diverse properties of the resulting derivatives, inorganic chemistry is closely associated with many methods of analysis. Older methods tended to examine bulk properties such as the electrical conductivity of solutions, melting points, solubility, and acidity. With the advent of quantum theory and the corresponding expansion of electronic apparatus, new tools have been introduced to probe the electronic properties of inorganic molecules and solids. Often these measurements provide insights relevant to theoretical models. Commonly encountered techniques are:
- Electron-spin resonance: ESR allows for the measurement of the environment of paramagnetic metal centres.
Also Check: What Does Abiotic Mean In Biology
The History Of Organic Chemistry
Into the early 19th century, naturalists and scientists alike observed a wide assortment of chemical compounds. These researchers noted critical differences existing between compounds that derived from living substances and those that did not. Chemists within this period of time recognized a fundamental yet inexplicable variance existing between types of different compounds. From these adept observations, the vital force theory of 1809 became a widely accepted conjecture.
Jons Berzelius proposed this vitalism theory. He was also the first individual to use the term organic chemistry, referring to the study of compounds originating from biological sources. The vital force theory proposed that a vital forceor a life forceexisted solely within the presence of organic materials, such as the bodies of living animals and plants. Under this belief, organic compounds were formed solely in living cells. Therefore, preparing such compounds in a laboratory was an impossibility.
Scientist Friedrich Wohler discarded this theory several decades later with his innovative synthesis method. Under controlled conditions, Wohler demonstrated how to obtain organic compounds in a laboratory. This in-vitro synthesis of organic matter proved to be a pivotal biochemistry discovery for further studies, conceptualizing the modern organic chemistry field.
Organic Compounds And Life
While most organic compounds encountered in chemistry are produced by living organisms, it’s possible for the molecules to form through other processes.
For example, when scientists talk about organic molecules discovered on Pluto, this doesn’t mean there are aliens on the world. Solar radiation can provide energy to produce organic compounds from inorganic carbon compounds.
Recommended Reading: Exponential Growth And Decay Worksheet Algebra 1
The History Of Inorganic Chemistry
Comprehensively, the field of chemistry revolves greatly around the existence of metals. Ancient techniques from alchemists in Asia and Europe ultimately lead to the development of this branch of science. While alchemists did not successfully change lead into valuable gold, their discovery of acids and bases, developmental methods of chemical reactions, and systematic research approach laid out the foundation for the periodic table.
The chemical nature of such substances did not become widely known until the 19th century, when scientists started to better understand organic compounds. Since then, inorganic chemists study the behavior of inorganic elements formed by non-living processes and work towards developing applicatory methodology or performing research on how these compounds can be effectively modified and utilized in industry.
Comparisons Between Organic And Inorganic Compounds
- In terms of abundance, organic compounds are more than inorganic compounds. That is because of the carbon atoms unique chemical ability to bond with multiple carbon atoms to form chains. There are over 19 million known compounds of carbon studied in organic chemistry. On the flip side, inorganic chemistry contains about 500,000 compounds.
- Organic compounds have lower boiling and melting points than inorganic compounds.
- Inorganic compounds are more soluble in water compared to organic compounds.
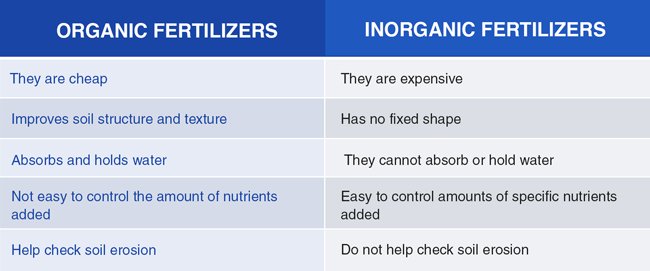
- Organic compounds result from the activities of living organisms. On the other hand, inorganic compounds result from naturally occurring processes or are manufactured in the laboratory.
- Due to the covalency exhibited by carbon, organic compounds do not form salts. On the other, inorganic compounds can form salts.
- All organic compounds have carbon atoms, while inorganic compounds have metal and other combined elements.
- The carbon-hydrogen bonds are found in organic compounds, but they are absent in inorganic compounds.
- Organic compounds are biological, while inorganic compounds are mineral in nature.
- All organic compounds from covalent bonds, whereas inorganic compounds can be both covalent and ironic.
- Organic compounds mainly contain carbon, oxygen, and hydrogen. By contrast, inorganic compounds generally lack carbon in their structure.
Examples of organic compounds and molecules
- Methane
- Sodium carbonate
How important are organic compounds?
Importance of inorganic compounds
Uses
Also Check: Segment Addition Postulate Find The Length Indicated
Difference Between Organic And Inorganic Chemistry
In a broad view, chemistry can be classified as a branch of physical science that explains the origin, structure and behaviour of matter and the change of matter from one form to another. Inorganic and Organic Chemistry are two different branches of chemistry that depend on the nature of compounds. The main difference between organic and inorganic chemistry is that ;inorganic chemistry is the study of inorganic compounds, whereas organic chemistry is the study of organic compounds.
This article explains,
1. What is Inorganic Chemistry?;;;; Definition, Reactions, Structure, Properties
2. What is Organic Chemistry?;;;; Definition, Reactions, Structure, Properties
3. What is the difference between Inorganic and Organic Chemistry?
Main Group Elements And Lanthanides
The mechanisms of main group compounds of groups 13-18 are usually discussed in the context of organic chemistry . Elements heavier than C, N, O, and F often form compounds with more electrons than predicted by the , as explained in the article on hypervalent molecules. The mechanisms of their reactions differ from organic compounds for this reason. Elements lighter than carbon as well as Al and Mg often form electron-deficient structures that are electronically akin to carbocations. Such electron-deficient species tend to react via associative pathways. The chemistry of the lanthanides mirrors many aspects of chemistry seen for aluminium.
Read Also: Math Caching Algebra 1 Answers
Differences Between Organic And Inorganic Compounds
Firstly, organic compounds are produced by and mostly associated with living organisms. They often result from human activities while non-living processes produce inorganic compounds. Therefore, it is common to find that organic compounds contain fatty acids, lipids, proteins, and enzymes found in the cells of living organisms while inorganic compounds contain salts and metals. Secondly, organic compounds contain metal atoms while the inorganic compounds do not have the metal atoms. Thirdly, organic compounds consist of carbon atoms together with hydrogen atoms forming hydrocarbons. On the contrary, inorganic compounds possess neither hydrogen nor carbon.
Finally, all organic compounds have carbon without exception. However, some few inorganic compounds contain carbon in them. For example, carbon monoxide has carbon atoms, however, it is not enough to classify it under organic compounds. The same case applies to carbon dioxide. Hence there is an exception to the general rule when it comes to inorganic compounds.
Different Types Of Chemistry
Fundamentally, chemistry is the study of matter and change. The way that chemists study matter and change and the types of systems that are studied varies dramatically. Traditionally, chemistry has been broken into five main subdisciplines:;Organic,;Analytical,;Physical,;Inorganic, and;Biochemistry. Over the last several years, additional concentrations have begun to emerge, including Nuclear chemistry, Polymer chemistry, Biophysical chemistry, Bioinorganic chemistry, Environmental chemistry, etceteras. All of these areas of chemistry are addressed in our classes here at UWL to some extent, and by the;research;interests of our faculty in the Chemistry Department. The following descriptions of the five major subdisciplines were written by several of our faculty members in their field of expertise. All of our faculty;members would be happy to elaborate, and/or discuss other aspects of chemistry that are not described below!
AnalyticalAnalytical chemistry;is the science of identification and quantification of materials in a mixture. Analytical chemists may invent procedures for analysis, or they may use or modify existing ones. They also supervise, perform, and interpret the analysis. Students concentrating in analytical chemistry often go on to work in forensics laboratories, environmental or pharmaceutical companies, work in, manage and/or design quality assurance procedures, pursue research, or teach in colleges and universities.
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
Main Differences Between Organic And Inorganic Compounds
Characteristics Of Inorganic Compounds
Most of the inorganic compounds have the capacity of conducting electricity. When the inorganic compounds are in the solid-state, they are poor conductors of electricity while in a liquid state, the inorganic compounds are very good conductors of electricity. All the electrons, in this particular phase, move around freely. And this particular movement of electrons is termed electricity.
Ionic bonds are particularly available in inorganic compounds which keep them in very close proximity. Hence they are packed densely and contain high boiling as well as melting points. Another feature of inorganic compounds is colored.
You May Like: Algebra 1 Eoc Answers 2015
Molecular Symmetry Group Theory
A central construct in inorganic chemistry is the theory of molecular symmetry. Mathematical group theory provides the language to describe the shapes of molecules according to their point group symmetry. Group theory also enables factoring and simplification of theoretical calculations.
Spectroscopic features are analyzed and described with respect to the symmetry properties of the, inter alia, vibrational or electronic states. Knowledge of the symmetry properties of the ground and excited states allows one to predict the numbers and intensities of absorptions in vibrational and electronic spectra. A classic application of group theory is the prediction of the number of C-O vibrations in substituted metal carbonyl complexes. The most common applications of symmetry to spectroscopy involve vibrational and electronic spectra.
Group theory highlights commonalities and differences in the bonding of otherwise disparate species. For example, the metal-based orbitals transform identically for WF6 and W6, but the energies and populations of these orbitals differ significantly. A similar relationship exists CO2 and molecular beryllium difluoride.
Understanding Organic And Inorganic Compounds
In relative terminology, the word organic refers to anything that derives from or relates to living matter. Conversely, its antonym inorganic relates to anything not deriving from or consisting of living matter. This definition holds true within the scientific field of biology, but modern chemistry is a diverse subject. The essential basis of chemistry revolves around a dynamic duonamely, that of organic and inorganic compounds. Scientifically, two distinct disciplines exist because of these divergent facets of matter-examining chemistrythat of organic chemistry and inorganic chemistry.
The primary difference between these opposite disciplines is their field of study. To generalize the variance, organic chemistry involves the study of living compounds containing carbon atoms, whereas inorganic chemistry involves the study of mainly non-carbon compounds obtained from non-living things. Scientists principally regard substances that do not fall directly into the definition of organic as inorganic.
Recommended Reading: Grade 6 Fsa Warm-ups Answer Key
Comparison Table Between Organic And Inorganic Compounds
| Parameter of Comparison | ||
|---|---|---|
| They are distinguished by carbon atoms which are present in them | Inorganic substances do not comprise carbon atoms | |
| Reactivity | They are considered to be more reactive and extremely flammable | They are naturally not volatile and are also not flammable |
| Physical state | These substances occur as gases, liquids, and solids. | They solely occur as solids |
| Occurrence | Organic substances are present predominantly in most living organisms | They majorly occur in non-living organisms |
| Melting and boiling points | High melting and boiling points are among the main aspects used to characterize organic substances. | Compared to organic substances, these are characterized by low boiling and melting points. |
Thermodynamics And Inorganic Chemistry
An alternative quantitative approach to inorganic chemistry focuses on energies of reactions. This approach is highly traditional and empirical, but it is also useful. Broad concepts that are couched in thermodynamic terms include redox potential, acidity, phase changes. A classic concept in inorganic thermodynamics is the Born-Haber cycle, which is used for assessing the energies of elementary processes such as electron affinity, some of which cannot be observed directly.
You May Like: Segment Addition Postulate Practice Answer Key
Examples Of Substances Under Scope Of Study
Examples of organic substances studied under organicchemistry include: sucrose, methane, DNA, Benzene, Cellulose, hair andfingernails, butter, enzymes, coal, Ethanol etc. In contrast, examples ofinorganic substances studied under organic chemistry include: Sodium chloride,Argon, Silver, Brass, Sulfur, Diamond, Carbides, phosgene, carbon disulfide,cyanides etc.
What Is The Difference Between Organic And Inorganic Compounds
March 25, 2020 By C. Hall
Wherever we look around, you can see both inorganic and organic compounds. When we go to supermarket, we see different items; some are organic and others are inorganic. What is the difference between organic and inorganic compounds?
Well, the chemical difference is not the one you hear on the news which distinguishes organic vegetables from non-organic ones. Guess what, both are made up of organic and inorganic compounds.
Lets say that the agriculture industry definition is not the same as the chemical definition. In chemistry, there is a major difference, which is well defined.
Telling the difference between organic and inorganic compounds is one of the main things you need to make clear while learning chemistry. If you are interested, learn more about thermodynamics and kinetics, another two of thee most important concepts in chemistry.
In this article we will explain it in detail, so at the end you will be able to differentiate both of types of chemicals without any difficulty. We will try to solve all your doubts about this eternal chemistry question!
In the early days, scientists separated organic and inorganic compounds on the fact that the first group was considered as a result of the activity of living beings, whereas the second group belonged to the processes unrelated to any way of life. Now there are much clearer definitions.
You May Like: What Kind Of Math Is On The Ged Test
What Is The Difference Between Organic Chemistry And Inorganic Chemistry
Organic chemistry is the field of chemistry that deals with the structure, properties, reactions and other facts about organic compounds whereas inorganic chemistry is the field of chemistry that deals with inorganic compounds. This is the key difference between organic and inorganic chemistry. Since most of the organic compounds are covalent compounds, we have to deal mainly with covalent compounds during the study of organic chemistry. However, most of the inorganic compounds are ionic compounds. Hence we have to deal with ionic compounds during the study of inorganic chemistry. Hence, this is a major difference between organic and inorganic chemistry.
Further differences are shown in the infographic of the difference between organic and inorganic chemistry.
History Of Organic Chemistry
Earlier, organic chemistry was seen as a branch of the natural processes that take place in a living organism. But in the early 1800s, Friedrich Wohler was the first human being to discover that organic compounds are the ones that are capable of getting synthesized from various minerals and also different non-organic materials that are present in a laboratory.
Recommended Reading: Lesson 1.7 Practice A Geometry Answers
Applications Of The Organic Chemistry
There is many application of Organic Chemistry, wider than the Inorganic Chemistry. It is a complex study and therefore offered as a different subject within Chemistry. Organic Chemistry talks about solvents, graphites, carbons and so on. It also has a wide range of applications and it is fascinating to see that so many substances such as milk, sugar, and the bread that we use in our daily life are made of the organic material. Apart from edible, there are other things such as belts, shoes, tires, computers, machines that also come under this category. There are various uses of Organic Chemistry and let us walk through each application one by one.
Medicine One place where you would find organic compounds in abundance is the medical store. Not all, but there is a wide variety of drugs that are made from the organic compounds. Organic compounds form a part of antibiotics, painkillers, anti-depressant, anesthetics, and so on. The organic compounds make the water of the medicine soluble as well as easily movable within the body tissues. With the study of Organic Chemistry, the experts look to improve the efficiency of drugs.
Pathophysiology of the disease
For the purpose of Synthesis