The Side Effects Sound Scary Which Ones Do I Need To Worry About Most
The most common misconception or concern I hear in my practice is that taking biologics will wipe out someones immune system, leading to infections, health problems and risk of death. When you take a drug that affects your immune system, you do have to keep an eye out for cancer and infections. So, before you start a biologic, you will be screened for cancer as appropriate for your age and sex, and for infections like tuberculosis and hepatitis. We also recommend everyone on these drugs gets the appropriate immunizations for their age, like flu and pneumonia shots, to reduce infection risk. But people live better and longer with these drugs than without. Major risks are associated far more with the disease and the consequences of untreated disease than these drugs. Biologics reduce the risks of premature death, increased heart disease and the need for joint surgery. Patients with uncontrolled RA are also at higher risk of infection, so controlling the arthritis can also reduce overall infection risk. On balance, you are much better off with treated disease than untreated.
Industry User Fees Only Make Up A Fraction
The post overstates the role of user fees in the FDA budget, though they are a significant portion.
About 45% of the FDA’s budget, or $2.7 billion, comes from industry user fees, according to;a fact sheet .;The other 55%, or $3.2 billion, comes from federal funding.;
A further breakdown of the agency’s total budget does show, depending on the department, industry fees can account for most of the funding, however.
For regulatory activities involving human drugs , 65%, or about $656 million, is funded;by industry user fees.;
For biologics, which includes vaccines;and represents 7% of the FDA’s total budget, industry fees pay about 40%, or around $337 million.;;
While there is a concern industry-based funding which increased;nearly 42% between the fiscal years 2017 and 2021; may;pose a conflict of interest, the FDA has said its drug approval decisions are independent of where the funding comes from.
“User fees provide instrumental funding for the FDAs independent review of medical products that make a difference in the lives of all Americans, without compromising the agencys commitment to scientific integrity, public health and regulatory standards, patient safety and transparency,” spokesperson Michael Felberbaum said in a statement to USA TODAY.;
Use Of Living Production Systems
- First, living systems must be kept alive 24/7 during the whole fermentation process. This also means that the cells must be revived from the frozen state that they were in during their storage in the MCB. Then, during culture, an adequate number of living cells must be kept alive and is considered as a CQA that can affect not only the total amount of biologic produced but also the amount of process-related impurities.
- Second, they must be kept happy, so they do great work. This involves providing our living production system the adequate nutrients in the correct proportions and, a friendly environment of an appropriate oxygen and carbon dioxide gas concentration, pH, and temperature. There is a range of parameters where the living system will produce at a high rate a highly qualitative product, and we need to keep it during the whole manufacturing process.
- Third, they must be kept healthy. This means erecting multiple barriers around the manufacturing process to protect the living system from the attack by adventitious agents during the production of the biologic. Chemical processes often prevent this kind of infections due to the unfriendly environment for bugs to develop. In the case of biomanufacturing, everything is done to make living production systems to grow fast and long. Adventitious agent can benefit fis environment too to grow.
Don’t Miss: How To Teach 4th Grade Math
Two: The Master Cell Bank
Step two is for the manufacturer to establish a master cell bank that supplies genetically identical cells for future products. Companies create cell banks by transferring the production cell line to a bioreactor. Though they may sound scary, bioreactors are simply vessels filled with a growth medium a broth with the required nutrients brewing in optimal conditions of temperature, pH, and oxygen concentration for cell growth.
The cells are left to simmer, or multiply for a few generations, creating hundreds of millions of identical copies. The manufacturer collects this slough and portions them into small vials. Each of the several hundred receptacles contains about a million cells. The vials are then frozen with liquid nitrogen, cooling them to -196 degrees Celsius. The deep freeze stops cell growth; In other words, if some future scientist thawed one of the vials in twenty years, she or he would find the cells inside exactly as they were at storagebarring apocalypse or someone tripping over the power strip. This stable longevity is key, as product consistency over the lifetime of the product is critical to drug safety.
Manufacturers typically divide the master cell bank for storage in three separate locations so that disaster in one place doesnt wipe out this important resource.
What Are Biologicals And Why Are They Important
- Case Studies
- Here
Biological crop protection products, also called biologicals, represent a broad category of plant protection products that are derived from living organisms.Growers use biologicals to complement chemical products in an integrated pest management program,or as stand-alone method, for protecting plants from disease, insect pests and competition from weeds. Here Robyn Kneen, Head Global Regulatory Affairs Biologics Bayer, explains more.
How do biologicals differ from;pesticides?
The biggest difference between biologicals andchemicals is that biologicals are made from living or;naturally occurring materials and chemicals are not. Biologicals can be chemically synthesized but nature-like in composition. Both types of products offer protection against a wide variety of plant diseases, insect pests, and weeds for the farmers who use them.
How do they fit into an IPM strategy?
Biologicals wont replace chemical crop protection products but they do complement each other when used with an effective IPM strategy. They can provide a more holistic approach for growers to maximize crop yields, improv equality and minimize pest resistance.
How are biological products regulated?
How do farmers ensure the responsible;use of biologicals?
In similar ways to chemical crop protection. Farmers can apply biologicals with the same equipment used for their chemical products directly to plants or to the soil in which the plants are grown, or as a seed treatment.
Also Check: Which Founding Contributors To Psychology Helped Develop Behaviorism
Number One: Establishing The Cell Bank
First things first. Biomanufacturing involves engineering a cell to produce a specific protein. Using well-established techniques, scientists transfer a gene encoding the desired protein into a production cell.; The two most commonly used production cells are E. coli bacterial cells and Chinese hamster ovary cells, or CHO cells. Once a manufacturer successfully manipulates a cell to produce said protein, the cells multiply. Scientists call these genetically identical cells the;production cell line.
The Daily Journal Of The United States Government
Legal Status
This site displays a prototype of a Web 2.0 version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPOs govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA’s archives.gov.
Legal Status
Don’t Miss: What Does Mole Mean In Chemistry
Race Is Real But Its Not Genetic
For over 300 years, socially defined notions of race have shaped human lives around the globebut the category has no biological foundation.
13 Mar 2020
Please note that this article includes an image of human remains.
A friend of mine with Central American, Southern European, and West African ancestry is lactose intolerant. Drinking milk products upsets her stomach, and so she avoids them. About a decade ago, because of her low dairy intake, she feared that she might not be getting enough calcium, so she asked her doctor for a bone density test. He responded that she didnt need one because blacks do not get osteoporosis.
My friend is not alone. The view that black people dont need a bone density test is a longstanding and common myth. A 2006 study in North Carolina found that out of 531 African American and Euro-American women screened for bone mineral density, only 15 percent were African American womendespite the fact that African American women made up almost half of that clinical population. A health fair in Albany, New York, in 2000, turned into a ruckus when black women were refused free osteoporosis screening. The situation hasnt changed much in more recent years.
Meanwhile, FRAX, a widely used calculator that estimates ones risk of osteoporotic fractures, is based on bone density combined with age, sex, and, yes, race. Race, even though it is never defined or demarcated, is baked into the fracture risk algorithms.
Simple Checks To Control Dust And Mist
- Note the settlement and spread of contamination on surfaces.
- Check the airflow indicator on the extraction system.
- Check for damage and leakage from the process.
- Speak to the operator and encourage reporting of any defects.
Case study
| A cook developed breathing problems after working with flour dust. She worked in a small, poorly ventilated room, with nothing to control her exposure to the flour dust. She became severely asthmatic and, after retiring early on health grounds, was awarded compensation.What the employer has done? The employer has since installed an extraction system to remove the flour dust and introduced new ways of working such as using a scoop to transfer flour, using sprinklers to spread flour and keeping the work area clean. The risk of other workers developing occupational asthma has been reduced. |
Read Also: How Do You Find The Difference In Math
Identification Of Mrm Options
Risk management options are control measures that risk managers may consider to manage the microbiological risks and prevent or control foodborne diseases. The identification of management options should be based on the consideration of the ability of the control measures to mitigate the risks to desired level of health protection and on the practical feasibility, acceptability, and consequences of the options. An MRA can help in determining the efficacy of different control measures in achieving the desired objectives.
One category of risk management options is legislation. For managing biological hazards, a number of options are possible, including:
Another risk management option is the establishment of a FSO for a particular food safety issue. This approach offers flexibility to industry to select appropriate control measures that meet the FSO.
For certain problems, the risk management option of choice is the education of food handlers and consumers in hygienic handing of food. This is perhaps one of the key control measures applicable to most biological hazards, and particularly important for the preparation of food for infants and young children.
Keith I. Crews, in, 2020
Biologics In Autoimmune Diseases
Some of the most commonly used biologics are used for autoimmune diseases, diseases in which the bodys immune system plays a role in abnormally attacking its own tissue. These include conditions like rheumatoid arthritis, psoriasis, Crohns disease, and others. Many of these particular therapies are FDA-approved to treat more than one type of autoimmune disease. In some cases, doctors may prescribe these treatments off-label if they havent undergone the full suite of studies needed for FDA-approval, but there is still good reason to think they might be effective.
Because biologics are often expensive and more difficult to administer, they are often given after you have tried another non-biologic type of therapy.
One of the most common types of modern biologic therapies for autoimmune disease is the TNF blocker. TNF blockers include the popular drugs etanercept , adalimumab , and infliximab . These drugs all block the downstream inflammatory effects of an immune molecule called TNF-alpha. They are FDA-approved for several different autoimmune diseases.
Other biologics have been developed to block the receptors for different immune molecules. Others were designed to target T cells, specific cells in the immune system. Some of these other biologics important in autoimmune disease include:
- Ustekinumab
- Abatacept
- Guselkumab
Another important biologic in autoimmune disease is interferon beta-1a , which is a key treatment for multiple sclerosis.
Also Check: What Are Dyes In Chemistry
What Is A Biosimilar
Most of us are aware that conventional small molecule drugs are marketed under specific brand names, being familiar with the generic versions of these same drugs. The US FDA requires generic medicines to perform the same way as brand-name medicines, stating that: A generic medicine is the same as a brand-name medicine in dosage, safety, effectiveness, strength, stability and quality, as well as in the way it is taken and the way it should be used.
In this situation, generic drugs can be considered an equal substitute for their brand name counterparts. In the USA, a reported 90% of all prescriptions are for generic drugs . In the UK, the Kings Fund reported that the prescription of generic drugs has saved the NHS around £7.1 billion since 1976 and made it possible to prescribe 490 million more items without an increase in total spending .
It is hoped that continued research could lead to a similar situation with the discovery, development and manufacture of biosimilars. However, unlike a generic drug, a biosimilar is not an exact copy of its biologic counterpart. The inherent nature of biological products means that all biologics even reference products show inter-batch variation.
Although biosimilars differ in their structure to their counterpart biologics, for a biosimilar to be biosimilar they must have no clinically meaningful differences from an existing FDA-approved reference product.
Biologics Stem From Live Cells
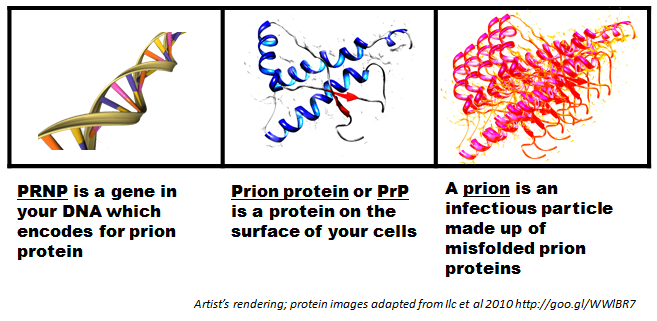
The strictest definition says biologic drugs only come from living systems or contain organic molecules, whereas small-molecule pharmaceuticals largely come from chemicals. Often, biologics are injected. If it’s swallowed, it’s probably not a biologic drug.
Because biologics use live materials, they must undergo extensive testing. From a scientific perspective, biologic drugs usually contain large-protein molecules that interact with specific cell receptors. Pharmaceuticals often are referred to as small-molecule drugs.
“It is difficult, and sometimes impossible, to characterize a complex biologic by testing methods available in the laboratory,” says the Biotechnology Innovation Organization. “And some components of a finished biologic may be unknown.”
Therefore, in biologics “the product is in the process,” the organization said in a post.
For example, AbbVie‘s Humira is the best-selling biologic drug in the world. In 2017, it brought in more than $18 billion in sales. Humira contains 1,300 amino acids. So replicating Humira exactly without AbbVie’s formula would be impossible.
Read Also: Geometry Wars 2 Smile Achievement
Increasing Competition For Biologics And Biosimilars
The U.S. biologics and biosimilars market is evolving rapidly, and the benefits for patient access and controlling health care costs will continue to grow over time as more medicines are introduced. As of November 2020, there are 18 biosimilars on the market in the U.S. competing against 7 reference biologics, with 10 additional FDA approved biosimilars due to come to market over the next several years.2 In FY 2020, there were 104 programs enrolled in the Biosimilar Product Development Program.3 In addition to products for oncology, immunology, and multiple sclerosis, since the successful addition of the transition products to the biosimilar pathway in March 2020 it is now possible for manufacturers to develop biosimilars for diseases including diabetes, respiratory distress syndrome, fertility conditions, Cushings syndrome, deep vein thrombosis, Gaucher disease and many more.4 The rich pipeline of potential biosimilar and interchangeable products currently in development as well as current market experience indicates that there is still significant potential for cost savings in the United States market.
2. PhRMA analysis of FDA, Biosimilar Product Information, https://www.fda.gov/drugs/biosimilars/biosimilar-product-information; IQVIA Institute, Biosimilars in the United States 2020 2024, ,
The Science Behind Biologics
Biologic therapies include wide range of medical products. Vaccines, blood products, and stem cell injections are examples of first-generation biologic therapies. However, when people talk about biologics they usually mean the second-generation biologic therapy drugs such as Humira, Remicade, and Enbrel.
Biologic response modifiers may be used to treat inflammatory autoimmune diseases, such as rheumatoid arthritis and ankylosing spondylitis. ReadBiologics: Basic Facts for Patients
Biologic therapies cannot be made using a simple chemical reaction, such as mixing ingredients together in a laboratory, the way conventional drugs are made. Instead, biologic therapies are made using living organisms, such as bacteria, yeast, and even mammalian tissue and cells.
You May Like: Math Caching Algebra 1 Answers
Why Is This Important
Prior to the implementation of these unique suffixes to the proper names of biological products, it was difficult to fully track adverse events for a specific manufacturers biologic if that product shared the same proper name of another biologic.1 The enhanced pharmacovigilance that is expected to result from these unique suffixes will allow for streamlined tracking of adverse events to a specific manufacturer, lot number, and/or manufacturing site.1 The unique suffix now allows for biological product differentiation.
There are concerns that many healthcare providers and patients assume that naming for biological products follows the same naming concepts as the naming of small-molecule drugs. If a small-molecule medication possesses the same generic name, they are more than likely interchangeable. However, this is not the case with biological products. A biological product possessing the same generic name does not infer interchangeability.2 The Purple Book includes guidance on all licensed biological products, including whether a biological product has been determined by the FDA to be interchangeable or if a biosimilar is interchangeable with the referenced product.9 The Purple Book was launched in 2014, is managed by the FDA, became searchable in February 2020, and is available at: purplebooksearch.fda.gov/.