What Is A Chemical Equation
nomenclature
A chemical equation is an expression of a chemical process. For example:
In this equation, AgNO3 is mixed with NaCl. The equation shows that the reactants react through some process to form the products . Since they undergo a chemical process, they are changed fundamentally.
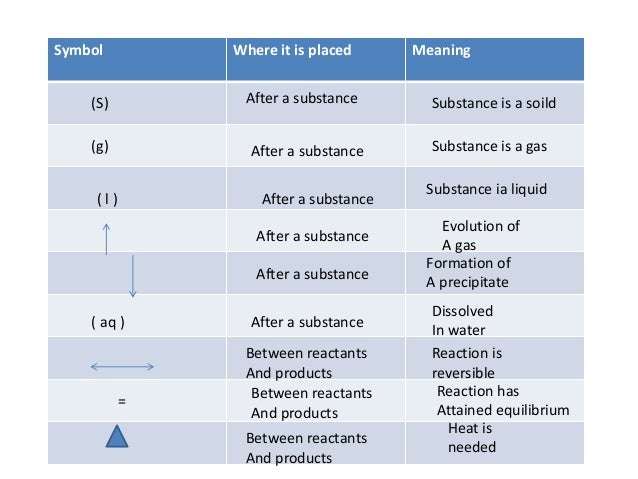
Often chemical equations are written showing the state that each substance is in. The sign means that the compound is a solid. The sign means the substance is a liquid. The sign stands for aqueous in water and means the compound is dissolved in water. Finally, the sign means that the compound is a gas.
Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of molecules, or the relative number of moles . If no coefficient is shown, a one is assumed.
On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, show what conditions need to be present for a reaction to occur. For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe2O3, a temperature of 1000 degrees C, and a pressure of 500 atmospheres for this reaction to occur.
The graphic below works to capture most of the concepts described above:
What Does Simp Mean In Slang
Urban Dictionarys top definition of a simp is someone who does way too much for a person they like. Other definitions on the crowdsourced online dictionary include a man who puts the hoes before the bros, and a guy that is overly desperate for women, especially if she is a bad person, or has expressed her
What Is An Aqueous Solution
What Is an Aqueous Solution in Chemistry?
What is an aqueous solution in chemistry? Well, to start, its the 4th state of matter. To a chemist, any substance is found in one of the 4 states of matter. You already know about 3 of them: solid, liquid, and gas. And, in chemistry, an aqueous solution is the 4th state of matter. So, you might say that aqueous solutions matter a lot, as they are literally an entire state, or configuration, of this stuff we call matter.
First thing first, I know there are other states of matter. To your physics teacher, the 4th state of matter would be called plasma. To your chemistry teacher, the 4th state of matter would be called an aqueous solution. A child playing with corn starch might think that oobleck is the 4th state of matter. Oobleck definitely would win the contest if it were based on how fun the name was to say. Yet, at least in chemistry, the 4th state of matter is an aqueous solution. It turns out that an aqueous solution is an extremely practical concept, perhaps unlike plasma and oobleck, which we dont really encounter so much in our everyday lives.
What Is an Aqueous Solution: The Simple Answer
So what is an aqueous solution? It simply means dissolved. Yes, the everyday concept of dissolving something. Thats it. The aqueous solution definition means simply that something has been dissolved in water. The aqueous symbol is .
Aqueous solution definition: something is dissolved in water
Aqueous symbol:
Recommended Reading: What Is Washing Soda In Chemistry
Types Of Arrows In A Reaction:
1. Right Arrow: The right arrow is the most prevalent arrow in a chemical reaction formula. The direction indicates the reaction’s direction.
- A chemical equation is an empirical representation of a chemical process.
- Base materials are represented on the left-hand side of the equation as reactants.
- The right-hand side of the equation contains the products, which are the reaction results.
- A chemical or physical transition is represented by the arrow.
- In chemical reaction formulae, the right arrow is by far the most prevalent arrow. The direction indicates the reaction’s direction.
What Is The Difference In And States In Reaction Mechanism
In these reactions between alkyl halide and KOH in different states the product is different only because of the change in state of KOH from aqueous and alcohol .
Can someone explain the mechanism which causes this drastic change?
Nucleophilic substitution always competes with Elimination reaction in such cases.
What is the difference between them?
In alcoholic solution the $\ce$ is basic enough to deprotonate a small amount of alcohol molecules ), thus forming alcohlate salts $\ce$. The alcoholate anions $\ce$ are not only more basic than pure $\ce$ but they are also bulkier . The higher bulkiness makes $\ce$ a worse nucleophile than $\ce$ and the higher basicity makes it better at $\mathrm2$ eliminations.
Mechanisms below:
You May Like: How To Find Percent Error In Chemistry
Types Of Chemical Reactions
Two or more reactants combine to make 1 new product.Examples:
A single reactant breaks down to form 2 or more products.Examples:
A single element replaces a similar element of an adjacent reactant compound.Example:
Two ionic compounds exchange ions, producing 2 new ionic compounds.Examples:HCl + NaOH NaCl + H2O
Balancing A Chemical Equation
Because the identities of the reactants and products are fixed, the equation cannot be balanced by changing the subscripts of the reactants or the products. To do so would change the chemical identity of the species being described, as illustrated in Figure \.
Original molecule H2O: if the coefficient 2 is added in front, that makes 2 water molecules but if the subscript 2 is added to make H2O2, that’s hydrogen peroxide.
The simplest and most generally useful method for balancing chemical equations is inspection, better known as trial and error. The following is an efficient approach to balancing a chemical equation using this method.
Steps in Balancing a Chemical Equation
Example \: Combustion of Heptane
Balance the chemical equation for the combustion of Heptane ).
Solution
Example \: Combustion of Isooctane
Don’t Miss: What Is The Formula Of Area In Physics
State Symbols And Phase Changes
|
Everything you need for better grades in university, high school and elementary. |
Learn with EaseMade in Canada with help for all provincial curriculums, so you can study in confidence. |
Instant and Unlimited HelpGet the best tips, walkthroughs, and practice questions. |
Make math click and get better grades! Join for Free
Get the most by viewing this topic in your current grade. Pick your course now.
Lessons
Free to Join!
Notes:
Properties Of Aqueous Solutions
Aqueous solutions often conduct electricity. Solutions that contain strong electrolytes tend to be good electrical conductors , while solutions that contain weak electrolytes tend to be poor conductors . The reason is that strong electrolytes completely dissociate into ions in water, while weak electrolytes incompletely dissociate.
When chemical reactions occur between species in an aqueous solution, the reactions are usually double displacement reactions. In this type of reaction, the cation from one reactant takes the place for the cation in the other reactant, typically forming an ionic bond. Another way to think of it is that the reactant ions “switch partners”.
Reactions in aqueous solution may result in products that are soluble in water or they may produce a precipitate. A precipitate is a compound with a low solubility that often falls out of solution as a solid.
The terms acid, base, and pH only apply to aqueous solutions. For example, you can measure the pH of lemon juice or vinegar and they are weak acids, but you can’t obtain any meaningful information from testing vegetable oil with pH paper.
Read Also: Different Sacred Geometry Patterns And Meanings
Definition Of Dissolve And The State Label In Chemical Equations
I am confused with the meaning of “” in chemical equations, and the meaning of “dissolved”. What is the actual meaning it is supposed to convey?
For instance, in the document “Preparing Bromine Water“, bromine molecules in bromine water are labelled $\ce}$.
What does dissolving in water mean? Must it be that a hydration shell is formed around the molecule? See:Dissolving of non polar gases in water
Even if no hydration shell is formed, small molecules just insert themselves into the space between solvent molecules, is it still called “dissolve”? It does seem more like being “dispersed”, as in a colloid.
Must “” mean that there is a hydration shell surrounding the molecule/ion?
For non-polar molecules dissolved in water, do we label it as “” in chemical equations? Must they form hydration shells?
The abbreviation in chemical equations or symbols simply means that this material is present in water as a homogeneous phase. The state symbol has nothing to do with the actual and true “structure” in solution. In short forget about hydration shell.
From IUPAC’s recommendations “Notation for states and processes, significance of the word standard in chemical thermodynamics, and remarks on commonly tabulated forms of thermodynamic functions” Pure & Appl.Chem., Vol.54, No.6, pp. 12391250, 1982.
$\ce$ would imply dissolved carbon dioxide in water .
$\ce$ would imply homogeneous solution of liquid bromine in water.
$\ce$: Salt dissolved in water.
A colloid is a heterogeneous phase.
How To Identify States Of Matter In A Chemical Formula
Chemical equations and the formulas of substances that appear in them are powerful recipes for laboratory experiments, and information about the state of matter of each reactant and product is vital. If you are only told that H2O is a reactant, you will not know whether to add ice — the solid form — or liquid water or steam — the gaseous form. Even if you are not physically performing an experiment, knowledge of the state of matter of each substance is necessary to fully understand the equation, and it can even help you identify the type of experiment. Since identifying states of matter is so important, it is fortunate that the process is very simple.
You May Like: Slader Linear Algebra 4th Edition
Empirical Formula And Molecular Formula
One can calculate the empirical formula from the masses or percentage composition of any compound. We have already discussed percent composition in the section above. If we only have mass, all we are doing is essentially eliminating the step of convertingfrom percentage to mass.
Example: Calculate the empirical formula for a compound that has 43.7 g P and 56.3 grams of oxygen.First we convert to moles:
Next we divide the moles to try to get a even ratio.
When we divide, we did not get whole numbers so we must multiply by two . The answer=P2O5
Calculating the molecular formula once we have the empirical formula is easy. If we know the empirical formula of a compound, all we need to do is divide the molecular mass of the compound by the mass of the empirical formula.It is also possible to do this with one of the elements in the formula simply divide the mass of that element in one mole of compound by the massof that element in the empirical formula. The result should always be awhole number.
Example: if we know that the empirical formula of a compound is HCN and we are told that a 2.016 grams of hydrogen are necesary to make the compound, what is the molecular formula? In the empirical formula hydrogen weighs 1.008 grams. Dividing 2.016 by 1.008 we see that the amount of hydrogen needed is twice as much. Therefore the empirical formula needs to be increased by a factor of two . The answer is: H2C2N2.
How To Write Balanced Chemical Equations

- Page ID
- 47502
- Explain the roles of subscripts and coefficients in chemical equations.
- Balance a chemical equation when given the unbalanced equation.
- Explain the role of the Law of Conservation of Mass in a chemical reaction.
Even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not disappear, nor do new atoms appear to form the products. In chemical reactions, atoms are never created or destroyed. The same atoms that were present in the reactants are present in the productsthey are merely reorganized into different arrangements. In a complete chemical equation, the two sides of the equation must be present on the reactant and the product sides of the equation.
You May Like: How To Get Rid Of E In Math
What Does Chemistry Mean In Love
Simply put, the feeling of chemistry with another person is that of connection. Its a draw to someone else that makes you want more of them. That doesnt have to be in a romantic relationship context, though that is the way we most often use the word. A few common types of chemistry are outlined below.
Use Of Arrow In Chemical Equations
When a reaction necessitates energy use, it is frequently shown above the arrow. If energy is provided to the reaction in the form of heat, a capital Greek letter delta is placed on top of the reaction arrow if energy is supplied in the form of light, hv is inscribed.
There are a wide variety of reactions possible in our everyday lives: Elements can form compounds , compounds can generate elements , or compounds can mix, split away, or reorganize to form new compounds.
Read Also: What Comes After Geometry In High School
Finding And Identifying States Of Matter
1
Locate the parentheses after the chemical formula, whether or not it is within the context of an equation.
2
Identify the parentheses as for solid, for liquid, for gas, or for aqueous solution. An aqueous solution is a substance dissolved in water.
3
If no parentheses follow the chemical formula, look for key words or phrases. These are especially helpful in the context of chemical reactions. For example, a precipitate is a solid, a combustion reaction produces water and carbon dioxide in their gaseous forms, and in solubility experiments, ions are in aqueous solution.
References
Aqueous Solution Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
An aqueous solution is any solution in which water is the solvent. In a chemical equation, the symbol follows a species name to indicate that it is in aqueous solution. For example, dissolving salt in water has the chemical reaction:
NaCl Na+ + Cl-
Although water is often called the universal solvent, it dissolves only substances that are hydrophilic in nature. Examples of hydrophilic molecules include acids, bases, and many salts. Substances that are hydrophobic do not dissolve well in water and tend not to form aqueous solutions. Examples include many organic molecules, including fats and oils.
When electrolytessuch as NaCl and KCldissolve in water, the ions allow the solution to conduct electricity. Nonelectrolytes like sugar also dissolve in water, but the molecule remains intact and the solution is not conductive.
Also Check: What Is Atp Stand For In Biology
How Will U Know If A Guy Loves U
If your boyfriend loves you, he will treat you with respect. That means that he listens to you and cares about whats going on in your life. He notices the little things that you like and goes out of his way to give them to you. He values you as a person, and he genuinely listens to your opinions.
Will It Dissolve
Whether or not a substance forms an aqueous solution depends on the nature of its chemical bonds and how attracted the parts of the molecule are to the hydrogen or oxygen atoms in water. Most organic molecules won’t dissolve, but there are solubility rules that can help identify whether or not an inorganic compound will produce an aqueous solution. In order for a compound to dissolve, the attractive force between a part of the molecule and hydrogen or oxygen has to be greater than the attractive force between water molecules. In other words, dissolution requires forces greater than those of hydrogen bonding.
Read Also: Is Ap Human Geography Hard