What Do Chemists Do
Chemists work in a variety of fields, including research and development, quality control, manufacturing, environmental protection, consulting and law. They can work at universities, for the government or in private industry, according to the ACS.
Here are some examples of what chemists do:
Research and development
In academia, chemists performing research aim to further knowledge about a particular topic, and may not necessarily have a specific application in mind. Their results, however, can still be applied to relevant products and applications.
In industry, chemists in research and development use scientific knowledge to develop or improve a specific product or process. For example, food chemists improve the quality, safety, storage and taste of food pharmaceutical chemists develop and analyze the quality of drugs and other medical formulations and agricultural chemists develop fertilizers, insecticides and herbicides necessary for large-scale crop production.
Sometimes, research and development may not involve bettering the product itself, but rather the manufacturing process involved in making that product. Chemical engineers and process engineers devise new ways to make the manufacturing of their products easier and more cost effective, such as increasing the speed and/or yield of a product for a given budget.
Environmental protection
Additional resources:
Converting Weight/weight Concentrations To Ppm
Recall the definition of parts per million in mass of solute per mass of solution units
1 ppm = 1 mg kg-1 = 1 g g-1
Some sample questions with worked solutions of converting w/w to ppm are given below.
Question 1.
What is its concentration in ppm?
Solution:
concentration = w/w = 0.033 g kg-1
mass of solute = 0.033 g
mass of solution = 1 kg
1 ppm = 1 mg kg-1 = 1 g g-1
mass of solution is in kilograms so mass of solute must be in milligrams
mass = 0.033 g = 0.033 g × 1000 mg/g = 33 mg
concentration = mass of solute ÷ mass of solution
concentration = 33 mg ÷ 1 kg = 33 mg kg-1
1 ppm = 1 mg kg-1 therefore 33 mg kg-1 = 33 ppm
0.033 g kg-1 = 33 ppm
Question 2.
What is its concentration in ppm?
Solution:
concentration is given as weight/volume
1 ppm = 1 g m-3 = 1 mg L-1 = 1 g mL-1
volume of solution is in milliltres so mass of solute must be in micrograms
mass = 0.0045 g = 0.0045 g × 106g/g = 4500 g
concentration = mass of solute ÷ volume of solution
concentration = 4500 g ÷ 150 mL = 30 g mL-1
Question 2.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Don’t Miss: Is Paris Jackson Related To Michael Jackson
Weight/volume And Volume/volume Basis
It is sometimes convenient to base concentration on a fixed volume, either of the solution itself, or of the solvent alone. In most instances, a 5% by volume solution of a solid will mean 5 g of the solute dissolved in 100 ml of the solvent.
Example \
Fish, like all animals, need a supply of oxygen, which they obtain from oxygen dissolved in the water. The minimum oxygen concentration needed to support most fish is around 5 ppm . How many moles of O2 per liter of water does this correspond to?
Solution
5 ppm means 5 grams of oxygen in one million mL of water, or 5 mg per liter. This is equivalent to / = 1.6 × 104 mol.
If the solute is itself a liquid, volume/volume measure usually refers to the volume of solute contained in a fixed volume of solution . The latter distinction is important because volumes of mixed substances are not strictly additive. These kinds of concentration measure are mostly used in commercial and industrial applications. The “proof” of an alcoholic beverage is the -percent, multiplied by two thus a 100-proof vodka has the same alcohol concentration as a solution made by adding sufficient water to 50 ml of alcohol to give 100 ml of solution.
Sample Molecular Weight Calculation
The calculation for molecular weight is based on the molecular formula of a compound . The number of each type of atom is multiplied by its atomic weight and then added to the weights of the other atoms.
For example, the molecular formula of hexane is C6H14. The subscripts indicate the number of each type of atom, so there are 6 carbon atoms and 14 hydrogen atoms in each hexane molecule. The atomic weight of carbon and hydrogen may be found on a periodic table.
- Atomic weight of carbon: 12.01
- Atomic weight of hydrogen: 1.01
molecular weight = + so we calculate as follows:
- molecular weight = +
- molecular weight of hexane = 72.06 + 14.14
- molecular weight of hexane = 86.20 amu
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
How To Calculate W/v
When making a solution in a lab, there are different ways to express the concentration. Some of the most common ways are listed below:
Here, the focus will be specifically on how to make solutions with their concentrations expressed as weight by volume or w/v.
Solved Examples For Mass Percent Formula
Q] Calculate the grams of NaOCl in 285 grams of a billboard bleach solution.
Solution During this problem the equation should be rearranged to unravel for the grams of solute.
We have,
Percentage of mass = x 100%
Gram of solute = /100
Gram of solute = /100 = 17.52 grams.
Therefore, the gram of solute is 17.52 grams.
Q] Whats the percent mass of 5g of caustic soda dissolved in 100g of water?
Solution: Steps are as follows
Therefore, the mass percent of 5g of caustic soda dissolved in 100g of water is 4.761%.
You May Like: Theory Of Everything Geometry Dash 2
Molecular Weight And Isotopes
Note, if you are working with specific isotopes of an atom, you should use the atomic weight of that isotope rather than the weighted average provided from the periodic table. For example, if instead of hydrogen, you are dealing only with the isotope deuterium, you use 2.00 rather than 1.01 for the atomic mass of the element. Ordinarily, the difference between the atomic weight of an element and the atomic weight of one specific isotope is relatively minor, but it can be important in certain calculations!
What Is The Meaning Of Ww Abbreviation In Chemistry
What is WW definition ?
WW definition is “Waste Water”.
What does WW mean in Chemistry?
WW mean that “Waste Water” for Chemistry.
What is WW acronym ?
WW acronym is “Waste Water”.
What is shorthand of Waste Water ?
The shorthand of “Waste Water” is WW.
What is the definition of WW acronym in Chemistry?
Definitions of WW shorthand is “Waste Water”.
What is the full form of WW abbreviation?
Full form of WW abbreviation is “Waste Water”.
What is the full meaning of WW in Chemistry?
Full meaning of WW is “Waste Water”.
What is the explanation for WW in Chemistry?
Explanation for WW is “Waste Water”.
What is the meaning of WW Abbreviation in Astrology ?
The site does not only include the meanings of the WW abbreviation in Chemistry. Yes, we know your main purpose is explanation of WW abbreviation in Chemistry. However, we thought that besides the meaning of the WW definitions in Chemistry, you can consider astrological information of WW acronym in Astrology. Therefore, the astrological explanation of each word in each WW abbreviation is also included.
WW Abbreviation in Astrology
- WW
You are very proud, determined, and you refuse to take no for an answer when pursuing love. Your ego is at stake. You are romantic, idealistic, and often in love with love itself, not seeing your partner as he or she really is. You feel deeply and throw all of yourself into your relationships. Nothing is too good for your lover. You enjoy playing love games.
Also Check: The Branch Of Chemistry That Involves The Study Of Substances
Introduction To The Water Ionization Constant Kw
Pure water undergoes auto-ionization or self-ionization by donating or accepting a proton between two molecules of water to form H3O+ and OH ions. This is also known as autoprotolysis or amphoteric nature of water.
The hydronium ion is a very strong acid and hydroxide ion is a very strong base. Thus they can associate again to form water molecule. So water molecules and the ions always stay in equilibrium. And the equilibrium lies to the left. Thus a very small amount of hydronium ions and hydroxide ions are found in water.
The equilibrium constant for this autoionisation of water is known as Kw. Thus
Kw =
Or simply Kw = .
Here we omit the concentration of water molecule which should stay as a denominator. The reason is, not much change in concentration is observed during this process.
How Molecular Weight Is Determined
Empirical data on the molecular weight of a compound depends on the size of the molecule in question. Mass spectrometry is commonly used to find the molecular mass of small to medium-sized molecules. The weight of larger molecules and macromolecules is found using light scattering and viscosity. Specifically, the Zimm method of light scattering and the hydrodynamic methods dynamic light scattering , size-exclusion chromatography , diffusion-ordered nuclear magnetic resonance spectroscopy , and viscometry may be used.
You May Like: Exponent Rules Worksheet 2 Answers
Examples Of Chemistry In A Sentence
chemistrychemistrychemistrychemistry clevelandchemistry Washington Postchemistry Detroit Free Presschemistry CNNchemistrySan Diego Union-Tribunechemistry Chronchemistry BostonGlobe.comchemistryUSA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘chemistry.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Going From W/v To Molarity
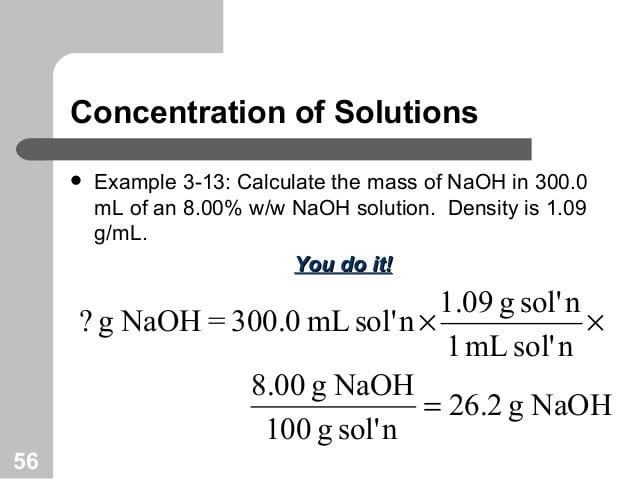
Say, however, your teacher gives you 21% solution of NaCl and asks you to find the molarity of this solution. How would you do that?
Begin by figuring out how many grams of solute you have in how much solution. Since the amount of solution you have will not change the concentration you can just assume 100 mL of solution.
So then:
Solving for the mass of solute you find:
This means that there are 21 grams of NaCl in every 100 mL of solution. To find molarity you will need to convert this number of grams into moles by using the molar mass of NaCl:
To find the molarity, you can divide the number of moles by the volume of the solute :
This means that a 21% w/v solution of NaCl is the same as a 3.6 solution of NaCl. In this way, you can convert between concentration designations.
Related Articles
Read Also: Age Problems Algebra
Percent By Weight Formula
Solutions can be described in other concentration besides molarity, normality or molality. Solutions are sometimes represented in terms of relative per cent concentration of solute in a solution. To determine the weight per cent of a solution, divide the mass of solute by mass of the solution and multiply by 100 to obtain per cent.
The per cent by weight formula can be expressed as
Example 1
Calculate how many grams of NaOH are required to make a 30.0% solution by using De-ionized water as the solvent.
Solution
Substitute the numbers that are known in the basic formula for per cent weight
X g of solute in 100g of solution = 30.0%
30.0 g of NaOH = X
Thus, 30 grams of NaOH must be dissolved in 70 grams of de-ionized water to make a 30% solution of NaOH in water. Furthermore, any amount of NaOH can be dissolved in de-ionized water in a 3:7 ratio to form a 30% solution of NaOH.
Example 2
Determine the % of a solution which has 25.0g of NaCl dissolved in100mL of De-ionized water.
Solution
Mass of solute = 25g
Mass of solvent = 100g
Therefore, mass of solution = mass of solute + mass of solvent = 100g + 25g = 125g
Mass % of NaCl in this solution = *100% = 20%
Therefore, mass % of NaCl in the solution is 20%
Molecular Weight Versus Molecular Mass
Molecular weight is often used interchangeably with molecular mass in chemistry, although technically there is a difference between the two. Molecular mass is a measure of mass and molecular weight is a measure of force acting on the molecular mass. A more correct term for both molecular weight and molecular mass, as they are used in chemistry, would be “relative molecular mass”.
You May Like: Who Are Paris Jackson’s Biological Parents
How To Convert From W/w% To Molarity
After reading a number of explanations about it after googling, I’m a bit confused on this. Some explanations are doing it using a solution density, others aren’t, though most are. On one site it’s showing different densities for a solution of a compound at different w/w% values. I’m now very lost as to how you convert from a w/w% to molarity. Can someone clarify?
Weight percent is essentially the same as :
$$\%~\mathrm = \mathrm$$
In order to get to the $\mathrm$ units of molarity, you have to convert by multiplying by the solution density, $\rho$, and dividing by the molecular weight of $\mathrm X$, $M_\mathrm$:
$$\mathrm \times \stackrel} \times \stackrel} = \mathrm$$
One key thing to remember is that $\rho$ is the density of the solution, not of the pure solvent. If you’re working with dilute solutions these two densities will be similar, but in concentrated solutions they will diverge.
Work Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The word “work” means different things in different contexts. In science, it is a thermodynamic concept. The SI unit for work is the joule. Physicists and chemists, in particular, view work in relation to energy:
Also Check: Geometry Segment Addition Postulate Worksheet
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Kw Increases With Increase Of Temperature
Autoionisation of water is an endothermic process. According to Le chateliers principle, if conditions are changed in a equilibrium process, the equilibrium will shift to such a direction where it can minimize the effect of the change of the condition. Thus if water is heated the equilibrium will shift to right to form more ions by absorbing extra heat as this is an endothermic process. According to the equation of Kw, if the concentration of ions increases the Kw increases. So we can say that Kw increases with the increase of temperature.
Don’t Miss: Eoc Fsa Warm Ups Algebra 1 Answers
S Per Million Calculations
Recall that, in general, concentration tells you how much solute is present in a solution.
concentration = amount of solute ÷amount of solution
A concentration in parts per million may refer to the mass of solute present in the volume of solution or it may refer to the mass of solute present in a mass of solution .
In SI units, w/w concentration would be given in kilograms of solute per kilograms of solution. So, a 1 part per million solution would be 1 kg of solute per 1 million kilograms of solution. And these masses are just too large to be useful in Chemistry laboratory. But we can divide the masses of solute and solution by 1 million to arrive at more useful units:
| 1 ppm |
ppm = mass of solute ÷ mass of solution
ppm = mass of solute ÷ mass of solution
You should practice rearranging the equations above in order to find mass of solute, volume of solution or mass of solution:
- To calculate mass of solute:
- To calculate volume of soution
- To calculate mass of soution
mass of solute = ppm × volume of solution
mass of solute = ppm × volume of solution
mass of solute = ppm × mass of solution
mass of solute = ppm × mass of solution
volume = mass of solute ÷ ppm
volume = mass of solute ÷ ppm
mass of solution = mass of solute ÷ ppm
mass of solution = mass of solute ÷ ppm