Anchoring Biomolecules On The Cell Surface Through Insertion Of Hydrophobic Chain Into The Cell Membrane
Although hydrophobic interactions are inherently weak in nature , they play a prominent role in many biological processes like lipid bilayer formation, protein folding, and proteinprotein recognition . In a seminal work by Dennis and coworkers using a lipid bilayer system, MSCs were treated with palmitated protein G where the palmitate chain was incorporated into the lipid bilayer via hydrophobic interactions and protein G provides generic binding sites for antibodies . In a proof-of-concept work, intercellular cell adhesion molecule-1 was conjugated onto MSCs, which enabled the cells to bind to ICAM-1, a critical adhesion molecule expressed on activated endothelium. These chemical approaches to tailor cell surfaces with functional ligands are broad and generic and are not limited to only MSCs and the ligands mentioned herein.
Figure 5.13. Diagrammatic representation of endothelial cell targeting using antibody-coated MSCs for tissue regeneration. A schematic representation of the membrane structure of AbICAM-1MSC. Targeting activated ECs expressing ICAM-1 abundantly in ischemic sites with AbICAM-1MSCs.
Figure 5.14. Schematic illustration of immobilization of urokinase on the islet surface. SH-PVA-alkyl was introduced to the cell surface by the hydrophobic interaction between alkyl side chains and the lipid bilayer of the cell membrane. Then, urokinaseMal was immobilized on the cell surface via thiolmaleimide bonding.
In , 2011
Main Difference Hydrophobic Vs Hydrophilic Molecules
Water is a well-known solvent for the dissolution of most of the compounds we know. But all compounds in nature do not mix with water. The substances that can mix with water are called hydrophilic substances the substances that cannot mix with water are known hydrophobic substances. This happens mainly due to the polarity of water molecules. Nonpolar compounds cannot dissolve in a polar solvent. Here, we should consider the fact like dissolves like. Polar compounds can dissolve in polar solvents. Nonpolar compounds dissolve in nonpolar solvents. Therefore, hydrophilic substances should be polar in order to dissolve in water. The main difference between hydrophobic and hydrophilic molecules is that hydrophobic molecules are nonpolar whereas hydrophilic molecules are polar.
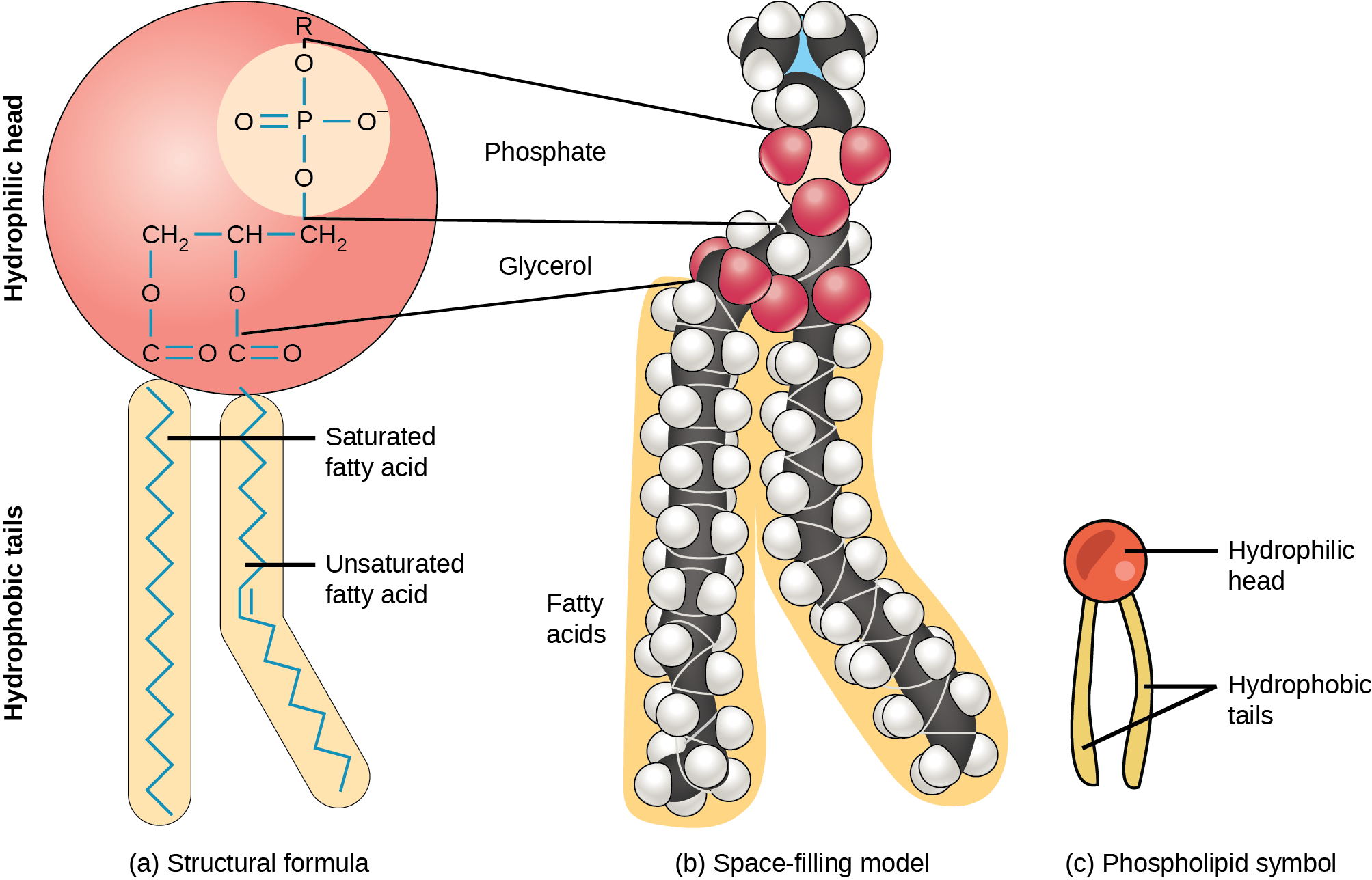
The Asymmetry Of The Lipid Bilayer Is Functionally Important
The compositions of the two monolayers of the in many membranes are strikingly different. In the human , for example, almost all of the lipid molecules that have cholineâ3N+CH2CH2âin their head group are in the outer monolayer, whereas almost all of the molecules that contain a terminal primary are in the inner monolayer . Because the negatively charged phosphatidylserine is located in the inner monolayer, there is a significant difference in charge between the two halves of the bilayer. We discuss in Chapter 12 how lipid asymmetry is generated and maintained by membrane-bound phospholipid translocators.
The asymmetrical distribution of phospholipids and glycolipids in the lipid bilayer of human red blood cells. The colors used for the phospholipid head groups are those introduced in Figure 10-12. In addition, glycolipids are drawn with hexagonal polar
Lipid asymmetry is functionally important. Many cytosolic proteins bind to specific head groups found in the cytosolic monolayer of the . The , for example, is activated in response to various extracellular signals. It binds to the cytosolic face of the , where phosphatidylserine is concentrated, and requires this negatively charged for its activity.
Some functions of membrane phospholipids in cell signaling. Extracellular signals can activate PI 3-kinase, which phosphorylates inositol phospholipids in the plasma membrane. Various intracellular signaling molecules then bind to these phosphorylated
Read Also: Geometry Segment And Angle Addition Worksheet
Example Question #: Hydrophobic Interactions
How do hydrogen bonds compare in strength to covalent bonds, ionic bonds, and London dispersion forces?
Possible Answers:
Stronger than covalent bonds, London dispersion forces, and ionic bonds
Weaker than covalent and ionic bonds, but stronger than London dispersion forces
Weaker than London dispersion forces and ionic bonds, but stronger than covalent bonds
Weaker than covalent bonds and London dispersion forces, but stronger than ionic bonds
Stronger than covalent and ionic bonds, but weaker than London dispersion forces
Correct answer:
Weaker than covalent and ionic bonds, but stronger than London dispersion forces
Hydrogen bonds are the strongest of the intermolecular forces. However, that strength is little in comparison the strength of intramolecular forces, such as ionic and covalent bonds. The strongest of the listed forces is covalent bonds, followed by ionic bonds, hydrogen bonds, and then finally London dispersion forces.
Hydrogen bonds are important in biochemistry because of the incredible effect that they have on life due to their relative strength. But remember, this strength is not nearly as as strong as the covalent and ionic bonds, which actually hold atoms within the same molecule together.
Note, hydrogen bonds can be either an intermolecular or an intramolecular force. A hydrogen bond is considered intramolecular if it is occurring between different molecules, and intermolecular if it is occurring within the same molecule.
The Definition Of Hydrophobic With Examples
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
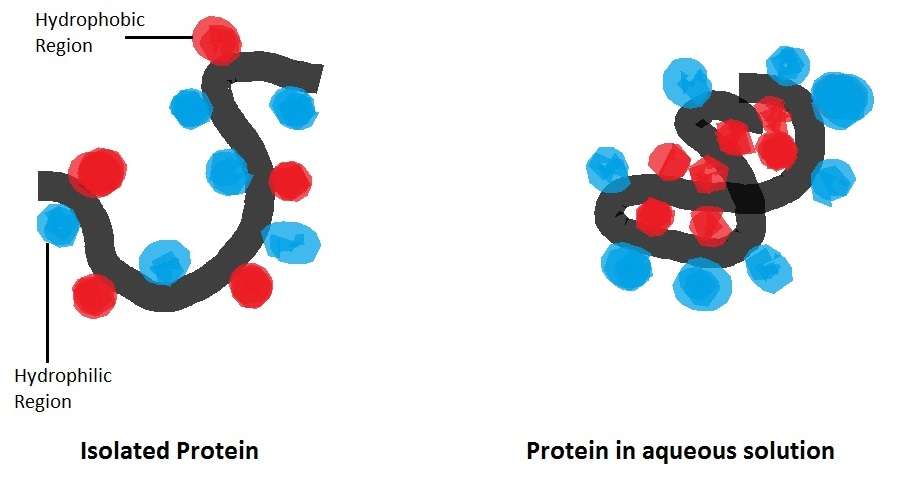
To be hydrophobic means to fear water. In chemistry, it refers to the property of a substance to repel water. It isn’t that the substance is repelled by water so much as it has a lack of attraction to it. A hydrophobic substance exhibits hydrophobicity and may be termed hydrophobic.
Hydrophobic molecules tend to be nonpolar molecules that group together to form micelles rather than be exposed to water. Hydrophobic molecules typically dissolve in nonpolar solvents .
There are also superhydrophobic materials, which have contact angles with water greater than 150 degrees. Surfaces of these materials resist wetting. The shape of water droplets on superhydrophobic surfaces is called the Lotus effect, in reference to the appearance of water on a lotus leaf. Superhydrophobicity is considered a result of interfacial tension and not a chemical property of matter.
Also Check: Ccl4 Geometric Shape
The Water Race: Hydrophobic & Hydrophilic Surfaces
Audience: High School
Nonpolar molecules that repel the water molecules are said to be hydrophobic molecules forming ionic or a hydrogen bond with the water molecule are said to be hydrophilic. This property of water was important for the evolution of life. Hydrophobic interaction plays the most critical roles in the formation of the lipid bilayer of the cell membrane and the folding of proteins and nucleic acids therefore, hydrophobic interaction is the foundation for the existence of life.
A self-assembled monolayer is a layer of organic molecules formed spontaneously on a solid substrate. One end of the organic molecule binds to the solid surface via a covalent bond while the other end points outwards. Because the exposed end of the SAM determines the surface properties of the SAM modified substrate, we can alter a hydrophobic surface into a hydrophilic surface by carefully selecting the SAM forming molecules.
This lesson requires the use of special chemicals which can be ordered from standard supply houses.
D: Introduction To The Hydrophobic Effect
Romanian Translation by Alexander Ovsov
Ukranian Translation by Vlad Brown
Now we can apply our understanding of o to the formation of micelles and bilayers. Remember, o = Ho – T So..
The diagram below shows the standard free energies of transfer of a hydrocarbon X from aqueous solution to a pure liquid hydrocarbon , x ——–> x .
o = ox – ox , which should be less than 0. In each graph, o is less than 0, and the value of o decreases in a linear fashion with increasing numbers of C in the alkyl chain. Notice the lines are unbelievably straight and parallel. Nature is speaking to us in these figures. By figuring out the surface area of the chains and the decrease in o with each added CH2 , one can calculate that the o decreases by 25 cal/sq. angstrom of hydrocarbon chain added.
Figure: Standard free energy of transfer of HC from aqeuous solution to a pure liquid hydrocarbon.
Figure: Transfer of Aliphatic Alcohols and Fatty Acids from Water to Pure Liquid Thermodynamic Parameters for Transfer of Aliphatic Alcohols From the Pure Liquid to Water.
| alcohol |
|---|
| +23 |
from Tanford, The Hydrophobic Effect
You May Like: What Is Elastic Force
Strength Of Hydrophobic Interactions
Hydrophobic interactions are relatively stronger than other weak intermolecular forces /Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Specific_Interactions/Van_Der_Waals_Interactions” rel=”nofollow”> Van der Waals interactions or Hydrogen bonds). The strength of Hydrophobic Interactions depend on several factors including :
Unfolding Degree And Adhesive Properties
Hydrophobic interactions induced by soy protein modification with various SDS concentrations have been proved to have different adhesive properties . With a pH of about 7.0, proteins modified with 0.5% and 1% SDS gave the highest gluing strength . Soy protein modified with SDBS, another anionic detergent, in a concentration range similar to that of SDS, presented similar gluing properties, as reported by Huang and Sun . The soy proteins modified with 1% SDS also had higher water resistance, showing no change in gluing strength after exposure to high relative humidity . Wood specimens glued with the 1% SDS modified protein adhesive had about a 10% reduction in shear strength after three water-soaking cycles .
TABLE 10.1. Adhesive strength of unfolded soy proteins with sodium dodecyl sulfate on cherry wood samples. The soaked strength refers to the strength after three-cycles of 48-hr water-soaking test.
| SDS Concentration |
|---|
J. Benavides, … J.A. Asenjo, in, 2011
You May Like: Cpm Algebra Homework Answers
Cold And Hot Transition States Ensembles
Cold and hot transition state ensembles were determined following a standard procedure based on the interpretation of -value analysis in terms of fraction of native contacts. Briefly, given a set of experimental values, a pseudo energy term has been added to the force field as the squared difference between experimental and simulated values in order to maximize the agreement with the experimental value while keeping the simulation stable. The value for a residue i is calculated from the fraction of native contacts that it makes in a conformation. Given two residues that are not nearest neighbours, the native contacts between them are defined as the number of heavy side-chain atoms within 0.65nm in the native structure. With this approach only values between 0 and 1 can be incorporated as structural restraints.
The different transition state ensembles were generated using simulated annealing. Each ensemble is the results of 300 annealing cycles, 150ps long, in which the temperature is varied between 272K or 323K and 500K. Only the structures sampled at the reference temperatures are retained for further analysis, resulting in TSE of ~1000 structures each.
The Strength Of Hydrophobic Interactions
hydrophobic interaction in protein is relatively stronger than other weak intermolecular forces .
What does the strength of Hydrophobic Interactions depend on?
In order of Effectiveness:
Also Check: What Influence Did Geography Have On The Development Of Greek Society
The Fluidity Of A Lipid Bilayer Depends On Its Composition
The fluidity of cell membranes has to be precisely regulated. Certain processes and activities, for example, cease when the bilayer viscosity is experimentally increased beyond a threshold level.
The fluidity of a depends on both its composition and its temperature, as is readily demonstrated in studies of synthetic bilayers. A synthetic bilayer made from a single type of changes from a liquid state to a two-dimensional rigid crystalline state at a characteristic freezing point. This change of state is called a phase transition, and the temperature at which it occurs is lower if the chains are short or have double bonds. A shorter chain length reduces the tendency of the hydrocarbon tails to interact with one another, and cis-double bonds produce kinks in the hydrocarbon chains that make them more difficult to pack together, so that the membrane remains fluid at lower temperatures . Bacteria, yeasts, and other organisms whose temperature fluctuates with that of their environment adjust the composition of their membrane lipids to maintain a relatively constant fluidity. As the temperature falls, for instance, fatty acids with more cis-double bonds are synthesized, so the decrease in bilayer fluidity that would otherwise result from the drop in temperature is avoided.
Cholesterol in a lipid bilayer. Schematic drawing of a cholesterol molecule interacting with two phospholipid molecules in one monolayer of a lipid bilayer.
Chaotropic Salts Influence On Equilibria
A chaotropic agent is a molecule in water or water/organic solvent solutions that can disrupt the hydrogen bonding and solvation of ions. Chaotropic solutes interfere with intramolecular interactions, such as hydrogen bonding, van der Waals forces, and hydrophobic effects, and as shown by formula , they affect the activity coefficient of the analytes. Chaotropic salts that dissociate in solution can produce a shielding of ionic charges. Also, since hydrogen bonding is stronger in nonpolar media, and salts increase the chemical polarity of the solvent, hydrogen bonding is affected. Chaotropic salts can be added in some mobile phases to influence the activity of the analytes and therefore the separation . Some inorganic ions can act as chaotropes with the increase in their disruptive character in the following order: H
J.A. McCune, O.A. Scherman, in, 2017
You May Like: How To Find Half-life
Vector Addition Of Bond Dipoles
If water were linear, the dipole moment vectors of the two bonds would cancel each other by vector addition, as shown in Figure 5 below:
When more than one polar bond is present in the same molecule, the polarity of one bond may cancel that of another. Thus the presence of polar bonds in a polyatomic molecule does not guarantee that the molecule as a whole will have a dipole moment. In such a case it is necessary to treat each polar bond mathematically as a vector and represent it with an arrow. The length of such an arrow shows how large the bond dipole moment is, while the direction of the arrow is a line drawn from the positive to the negative end of the bond. Adding the individual bond dipole moments as vectors will give the overall molecular dipole moment.
Figure 5: The simplest arrangements of equivalent bonds around a central atom which produce a resultant dipole moment of zero: linear trigonal. The two right-hand bonds cancel the left-hand bond. Tetrahedral. The three right-hand bonds cancel the left-hand bond.
Physical Properties And Molecular Structure
First, look at the melting points and boiling points of substances in the table below. Surprisingly, they are not related in any way to the molecular weights. This makes sense if you remember that molecular weight is a nuclear property. Melting and boiling involve breaking bonds between molecules, so like all bonding, they involve electronic structure. They depend on the size of atoms and molecules, but by size we mean volume, not mass.
Second, notice that the small water molecule is exceptional. It has the largest dipole moment, and a boiling point that is over 100°C higher than molecules of similar size.
Molecules of very low boiling points have zero polarity, indicated by the molecular dipole moment in debyes
The molecular dipole moments determine all the biological properties mentioned above, so we must explain why water is so polar, and methane, CH4, is a nonpolar molecule, even though it contains polar bonds.
| Molecule |
|---|
| Used to reduce “bends” |
| CH4 |
| acid rain |
Don’t Miss: Half Life Equations Chemistry
Thermodynamics Of Hydrophobic Interactions
When a hydrophobe is dropped in an aqueous medium, hydrogen bonds between water molecules will be broken to make room for the hydrophobe however, water molecules do not react with hydrophobe. This is considered an endothermic reaction, because when bonds are broken heat is put into the system. Water molecules that are distorted by the presence of the hydrophobe will make new hydrogen bonds and form an ice-like cage structure called a clathrate cage around the hydrophobe. This orientation makes the system more structured with an decrease of the total entropy of the system therefore \.
The change in enthalpy ) of the system can be negative, zero, or positive because the new hydrogen bonds can partially, completely, or over compensate for the hydrogen bonds broken by the entrance of the hydrophobe. The change in enthalpy, however, is insignificant in determining the spontaneity of the reaction because the change in entropy ) is large.
According to the Gibbs Energy formula
with a small unknown value of \ and a large negative value of \, the value of \ will turn out to be positive. A positive \ indicates that the mixing of the hydrophobe and water molecules is not spontaneous.
Interactions Dominated By London Dispersion Forces
London dispersion forces have often been overlooked in solution-phase chemistry, for the reasons discussed in previous sections. In addition, a number of well-known interactions between molecules that are dominated by London dispersion forces are known by different names. This section focuses on such molecular interactions in which London dispersion forces secretly play an important role.
3.1Hydrophobic interactions
Hydrophobic interaction is a term frequently used in biochemistry to describe the attractive interaction between the hydrophobic parts of a system these interactions are the result of London dispersion. Although the exclusion of water from hydrophobic pockets is caused both by entropic contributions and the preference of the water molecules to interact with each other through favorable hydrogen bond formation, the attraction between the hydrophobic parts is the result of London dispersion forces. These attractive forces play a significant role in the stabilization and folding of proteins.25
3.2 Stacking
Fig. 3. Stacked , T- shaped , and displaced stacked geometries of the benzene dimer.
3.3Aurophilicity
Fig. 4. Schematic representation of aurophilicity resulting from the small atomic radius and large number of electrons present in gold atoms.
3.4Halogen/chalcogen bonding
Fig. 5. Interaction of halomethane with formaldehyde.
Debjit Dutta, … Praveen Kumar Vemula, in, 2014
Recommended Reading: Punchline Bridge To Algebra Worksheets