What Does Aromatic Really Mean
The content of this page is reasonably well covered in the Ouellette textbook, 2/e, Ch 5 especially Sections 5.2-3. Thus this page is now a secondary supplement, with more examples, for my Intro Organic/Biochem with Ouellette. Students in my class using Ouellette are responsible for this material at the level covered in the textbook and class materials this page may be a useful supplement, but it is not essential.
This page was originally written for use with the Bettelheim textbook, 6/e. In Ch 14 Bettelheim presents aromatic compounds, but without a clear sense of what aromatic really means. This page was written to try to fill that gap. Textbook references below are to the Bettelheim book.
What is an “aromatic” compound? It is common to start by saying that aromatic compounds are compounds related to benzene. In fact, that is about as much as the Bettelheim textbook says about the nature of aromaticity. However, as you go on in organic chemistry you will find a variety of compounds called aromatic, even though they are not so obviously benzene derivatives.
Defining aromatic in terms of benzene is a useful start in an introductory course. As we will see here, it is not easy to give a more complete definition that is satisfying in an introductory course. But let’s try…
One simple example is pyridine, C5H5N, shown below:
| This example is simple enough, because it is very similar to benzene in structure. |
Acidity Of Aromatic Compounds
But now look at the anion derived from this chemical — the ion that would result if this chemical behaved as an acid, and gave off an H+ this ion is also shown below. Note that it now has six electrons in p orbitals perpendicular to the ring — very similar to pyrrole, above. If pyrrole is aromatic, then maybe this ion should be? Yes, it is. A manifestation of this is that the parent compound, cyclopentadiene, is a rather “strong” acid — by the standards of H attached to hydrocarbons. The acidity of cyclopentadiene is due to the stabilization of the resulting anion. Ka for cyclopentadiene is about 10-16. That certainly isn’t strong compared to compounds commonly discussed as acids, but it is 1029 times stronger than for the non-cyclic form of this molecule.
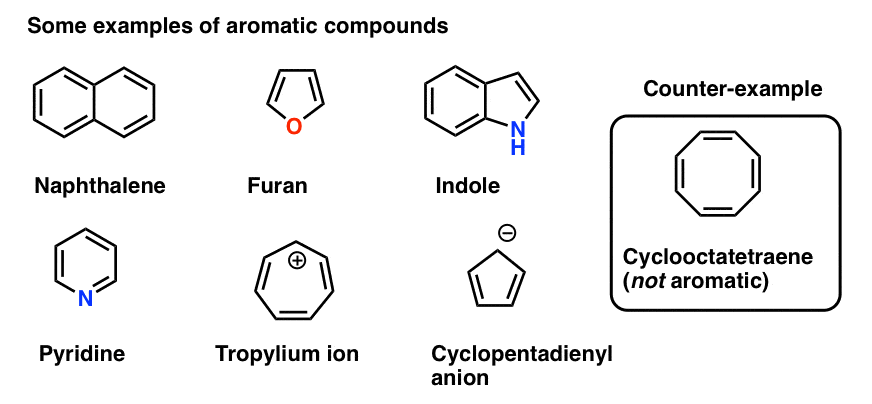
| An interesting example is 1,3,5,7-cyclooctatetraene , C8H8, shown at the left. At first glance, it would seem to be similar to benzene, except with a larger ring. But it is definitely not aromatic. Its chemical behavior is what you would expect for an alkene, and its shape is not planar, but is “tub-shaped”, as shown to the right. |
| cyclooctatetraene dianion |
Fused Aromatics And Polycyclics
Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms . Examples are naphthalene, anthracene, and phenanthrene. In fused aromatics, not all carboncarbon bonds are necessarily equivalent, as the electrons are not delocalized over the entire molecule. The aromaticity of these molecules can be explained using their orbital picture. Like benzene and other monocyclic aromatic molecules, polycyclics have a cyclic conjugated pi system with p-orbital overlap above and below the plane of the ring.
Read Also: What Is Visual Information Processing In Psychology
Iupac Nomenclature Of Aromatic Compounds
Earlier, most of the compounds with the same structural formula were known by different names depending on the regions where they were synthesized. This naming system was very trivial since it raised a lot of confusion. Finally, a common naming system enlisting standard rules was set up by IUPAC for the naming of compounds. This method of naming is IUPAC naming or IUPAC nomenclature.
IUPAC nomenclature of aromatic hydrocarbons is explained below:
1. According to IUPAC nomenclature of substituted aromatic compounds, the substituent name is placed as a prefix to the name of aromatic compounds. For example, a benzene ring attached to a one-nitro group is named as nitrobenzene.
2. When more than one similar substituent group is present in the ring, they are labelled with the Greek numerical prefixes such as di, tri, tetra to denote the number of similar substituent groups attached to the ring. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring, it is named 1,2-dibromobenzene.
3. When different substituted groups are attached to the aromatic compounds, the substituent of the base compound is assigned number one and then the direction of numbering is chosen such that the next substituent gets the lowest number. Substituents are named in alphabetical order. For example: when chloro and nitro groups are attached to the benzene ring, we first locate the chloro group then nitro groups.
Traditional Approaches To The Study Of Aromatic Hydrocarbon Metabolic Pathways
Biodegradation of aromatic hydrocarbons has been extensively studied , . Numerous bacterial strains have been isolated for the ability to aerobically degrade a variety of aromatic hydrocarbons. Microbial cells cultivated on aromatic hydrocarbons often exhibit the induction of enzymes involved in metabolic pathways . The bacterial degradation of aromatic hydrocarbons consists of many reaction steps, which have often been broadly separated into peripheral and central pathways . Peripheral pathways convert a large proportion of different aromatic hydrocarbons into a limited number of key central intermediates, such as catechol and protocatechuate. They are normally initiated by dioxygenases, often called Rieske nonheme iron ring-hydroxylating oxygenases , catalyzing the introduction of two atoms of oxygen into aromatic hydrocarbons to form cis-dihydrodiols . In the central pathway, these dihydroxylated intermediates are metabolized by enzymes that cleave the aromatic rings via ortho and meta pathways, which are then further degraded into TCA cycle intermediates.
You May Like: Algebra 1 Eoc Test Answers
Are All Aromatic Compounds Cyclic
Cyclic compounds may or may not be aromatic benzene is an example of a cyclic aromatic compound, while cyclohexane is non-aromatic. Organic compounds that are not aromatic are known as aliphatic compounds, but only aromatic rings are especially stable.
For a detailed discussion on the nomenclature of alkanes, alkenes, and alkynes, download BYJUS the learning app.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
All The Way From P To S
After first revealing the aromatic nature of some metal clusters, researchers were eager to dig deeper, finding many that they claimed were not only but also aromatic. Some compounds, like Wang and colleagues aluminium cluster, even showed both types of aromaticity, though this remains heavily debated. Nics and Gimic calculations do not agree in the type of electrons that cause the ring current, with the former pointing towards and contributions and the latter towards mostly , explains Florian Weigend, a quantum chemist at the University of Marburg.
But in clusters, valence electrons delocalise over the entire molecule, which doesnt necessarily make them aromatic. Along the metalmetal interaction axis there should be some kind of electron dynamics more so than in carbon compounds with defined covalent bonds, Dehnen explains. When non-localisable electron density was first observed in boron cages, it was a spectacular discovery, so it is understandable why the term 3D aromaticity was created, she says. Of course, there is aromaticity, but dont call everything made of metal atoms that has delocalised orbitals aromatic.
as does this 12-membered bismuth ring
are starting to realise that there is some chemical power that we can harness from this simple concept, says Wu.
You May Like: Renate Blauel 2013
Back To The Textbooks
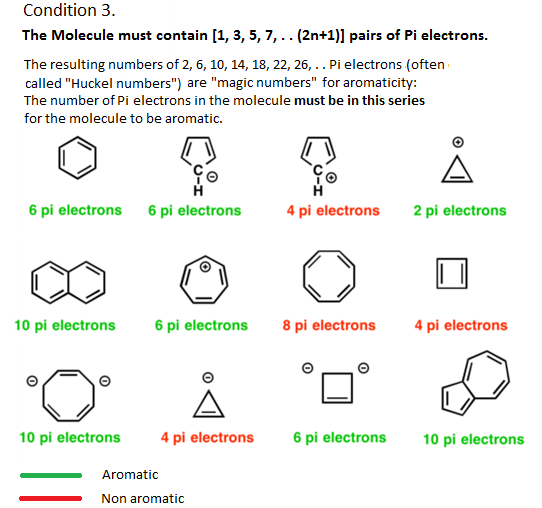
Many chemists first come across the concept of aromaticity in their undergraduate textbook or first-year lectures. According to these, all thats needed to identify aromaticity are four easy rules: a molecule must be cyclic, flat, have a continuous ring of p-orbitals and 4n+2 -electrons .
The last one, Hückels rule, is probably the most memorised of them all. It was developed by German physical chemist Erich Hückel in 1931 as part of the simplified quantum-mechanical calculations he used to deal with unsaturated organic molecules. He noticed that having 4n+2 -electrons gave aromatic molecules a filled molecular orbital shell, equivalent to the filled atomic orbital shell that gives noble gases their extraordinary stability. Hückels rule remained obscure until almost two decades later, when chemists first used it to validate tropones aromaticity.
There is no magic in having six electrons
But over the course of the 20th and 21st centuries, scientists discovered that these rules dont apply for all but a small subset of aromatics. In fact, the most archetypal of all aromatics benzene is not representative of the majority of aromatic molecules, wrote Wu.
What Is Aromatic In Organic Chemistry
organic chemistryaromaticityaromatic
. Moreover, what does it mean to be aromatic?
1 : of, relating to, or having a smell or odor. 2 of an organic compound : characterized by increased chemical stability resulting from the delocalization of electrons in a ring system containing usually multiple conjugated double bonds compare alicyclic, aliphatic. aromatic.
Beside above, what is Huckel rule explain with example? Because a closed bond shell of electrons defines an aromatic system, you can use Hückel’s Rule to predict the aromaticity of a compound. For example, the benzene molecule, which has 3 bonds or 6 electrons, is aromatic.
One may also ask, what is aromaticity with example?
In organic chemistry, the term aromaticity is used to describe a cyclic, planar molecule with a ring of resonance bonds that exhibits more stability than other geometric or connective arrangements with the same set of atoms. For example benzene ring C6H6.
What does aromatic mean LGBT?
An aromantic is a person who experiences little or no romantic attraction to others. The aromantic attribute is usually considered to be innate and not a personal choice, just as the lack of sexual attraction is innate to asexuals.
Read Also: Paris Jackson Biological Father
Choose The Right Synonym For Aromatic
Adjective
odorous, fragrant, redolent, aromatic mean emitting and diffusing scent. odorous applies to whatever has a strong distinctive smell whether pleasant or unpleasant. odorous cheeses should be tightly wrappedfragrant applies to things with sweet or agreeable odors. a fragrant rose redolent applies usually to a place or thing impregnated with odors. the kitchen was redolent of garlic and tomatoes aromatic applies to things emitting pungent often fresh odors. an aromatic blend of tobaccos
More Than Just An Academic Argument
What we are doing is trying to find connections between aromaticity and molecular properties that have real-world meaning, like HomoLumo gap or ionisation potential, which are important for functional materials, says Poranne. In 2018, she discovered that by defining a few simple building blocks and combination rules, she could predict the shape of Nics scans in polycyclic aromatic hydrocarbons. She and her team have now expanded this method to predict what happens to these molecules in the excited state when aromatics become antiaromatics and vice versa.
Alonso and her team found that certain porphyrin-type molecules can switch between aromatic and antiaromatic Hückel as well as Möbius aromatic structures. Since each of these forms has different conductivity, they could find use as switches in single molecule electronics and thermoelectric devices.
Weve evolved past the stage of asking what aromaticity means
With aromaticity now firmly past being purely an academic argument, is overhauling the definition even needed? Solà thinks so, particularly when it comes to borderline systems like metal clusters. In 2018, he closely examined 19 aromaticity rules, taking the first steps to combine and connect them with the eventual goal to find a single aromaticity theory that unifies them all.
Katrina Krämer is a science correspondent for Chemistry World
Recommended Reading: Exponent Rules Worksheet Algebra 2
Energetic Measure Of Aromaticity
Already in the nineteenth century, it was known that aromatic compounds are much more resistant to chemical reactions than their acyclic analogues . First quantitative description of aromaticity was proposed in 1933 by introduction of a thermodynamic term, namely the resonance energy, RE i.e. the energy by which the aromatic compound is more stable than its virtual olefinic analogue. In the case of benzene, this analogue is a virtual compound with three single and three double bonds. Estimated RE for benzene amounts to 36 kcal/mol. A very similar value was experimentally determined by Kistiakowsky et al. through calorimetric measurements of heats of hydrogenation of benzene and cyclohexene .
Later, the term RE was replaced by more precisely defined aromatic stabilization energy which is estimated by the use of either isodesmic or more precise homodesmotic reactions. The latter is defined as a virtual reaction leading to products with the same number of CH bonds and the same numbers of atoms in the appropriate hybridization states .
Table 1 Types of bonds for homodesmotic reaction shown in Scheme 1
Here the problem of an appropriate reference for the stability of aromatic molecules arises. Table 2, data taken from , shows that ASE values depend dramatically on the choice of the computation method of computation and of the selection of the reference system .
Table 2 Stabilization energiesa of ISODESMIC and reactions:
Aromatic Rings In Biology
Aromatic rings can be found in amino acids, DNA, and even the electron transport chain.
There are 3 amino acids that have aromatic rings: tryptophan , tyrosine , and phenylalanine . They appear frequently in protein-protein binding sites on proteins because of cation-pi interactions. Aromatic rings in amino acids can interact with positively charged amino acids. A cation-pi interaction is formed, which keeps these two groups close together.
Purines and pyrimidines are bases that show up in DNA and RNA. Pyrimidines are heterocyclic aromatic compounds. Examples of these are cytosine, thymine, and uracil. As heterocyclic compounds, the other element present in their structure is nitrogen. Alternatively, these are known as nitrogenous bases. Purines such as adenine and guanine are polycyclic aromatic compounds. Like pyrimidines, purines have nitrogen within their structure. They are also considered nitrogenous bases.
Aromatic molecules present in the electron transport chain are ubiquinone, NAD+, and FAD. All three of them can easily lose or gain electrons without compromising stability thanks to their aromatic rings. They are considered to be excellent electron carriers.
Ubiquinone acts as a carrier within the electron transport chain. It can accept two electrons which turns itself into ubiquinol. As it travels through the electron transport chain, it is oxidized and becomes ubiquinone once again.
Don’t Miss: Segment And Angle Addition Worksheet
Which Electrons Count As Pi Electrons And Which Do Not
That seems straightforward enough. However, complications can arise when we have atoms in the ring which both participate in pi bonding and also have a lone pair. For example,
- how do we count electrons in the benzene anion or pyridine? Do we count the lone pair electrons as Pi electrons, giving a total of 8? Or do we ignore them?
- What about furan which has two lone pairs on oxgyen?
- What about pyrrole, with its lone pair on nitrogen, or imidazole, with two nitrogens?
In order to answer these questions, its important to remind ourselves of how p orbitals contribute to aromaticity in benzene.
In benzene, each p orbital is arranged at right angles to the plane of the ring. Each p orbital contains a single electron. We can verify the total number of pi electrons in benzene by counting the pi bonds: 3 pi bonds times two electrons = 6 pi electrons total.
Note that the C-H bonds are at 90° to the pi system. If there was a lone pair where the C-H bond is, then it wouldnt be able to interact with the pi system at all. Which brings us to.
Aroma Compounds And Their Odors
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
An odor or odour is a volatile chemical compound that humans and other animals perceive via the sense of smell or olfaction. Odors are also known as aromas or fragrances and as reeks, stenches, and stinks. The type of molecule that produces an odor is called an aroma compound or an odorant. These compounds are small, with molecular weights less than 300 Daltons, and are readily dispersed in the air due to their high vapor pressure. The sense of smell can detect odors are extremely low concentrations.
Also Check: Is Paris Jackson Related To Michael Jackson
Some Examples Of Aromatic 5
Some molecules with five-membered rings can also present ambiguities.
The cyclopentadiene anion has a lone pair on one of the carbons. Can this lone pair contribute to the pi system?
Since that carbon is not involved in any pi-bonding, the answer is yes.
The total number of pi electrons for the cyclopentadiene anion equals 2 plus the 4 electrons in the two pi bonds, giving us a total of 6. This is a Hückel number and the cyclopentadiene anion is in fact aromatic.
A similar situation arises for pyrrole. The nitrogen bears a lone pair but is not involved in a pi bond . Therefore it can contribute to the pi system and this gives us a total of 6 pi electrons once we account for the 4 electrons from the two pi bonds.
A curious case isfuran, where the oxygen bears two lone pairs. Does this mean that furan has 8 pi electrons? No!
Why not? Because as we noted above, each atom can contribute a maximum of one p-orbital towards the pi system. In furan, one lone pair is in a p orbital, contributing to the pi system the other is in the plane of the ring. This gives us a total of 6 pi electrons. Furan is aromatic. .
Finally there is imidazole, which has two nitrogens. One nitrogen is not involved in a pi bond, and thus can contribute a full lone pair the other is involved in a pi bond, and the lone pair is in the plane of the ring. This also gives us a total of 6 pi electrons once we account for the two pi bonds.