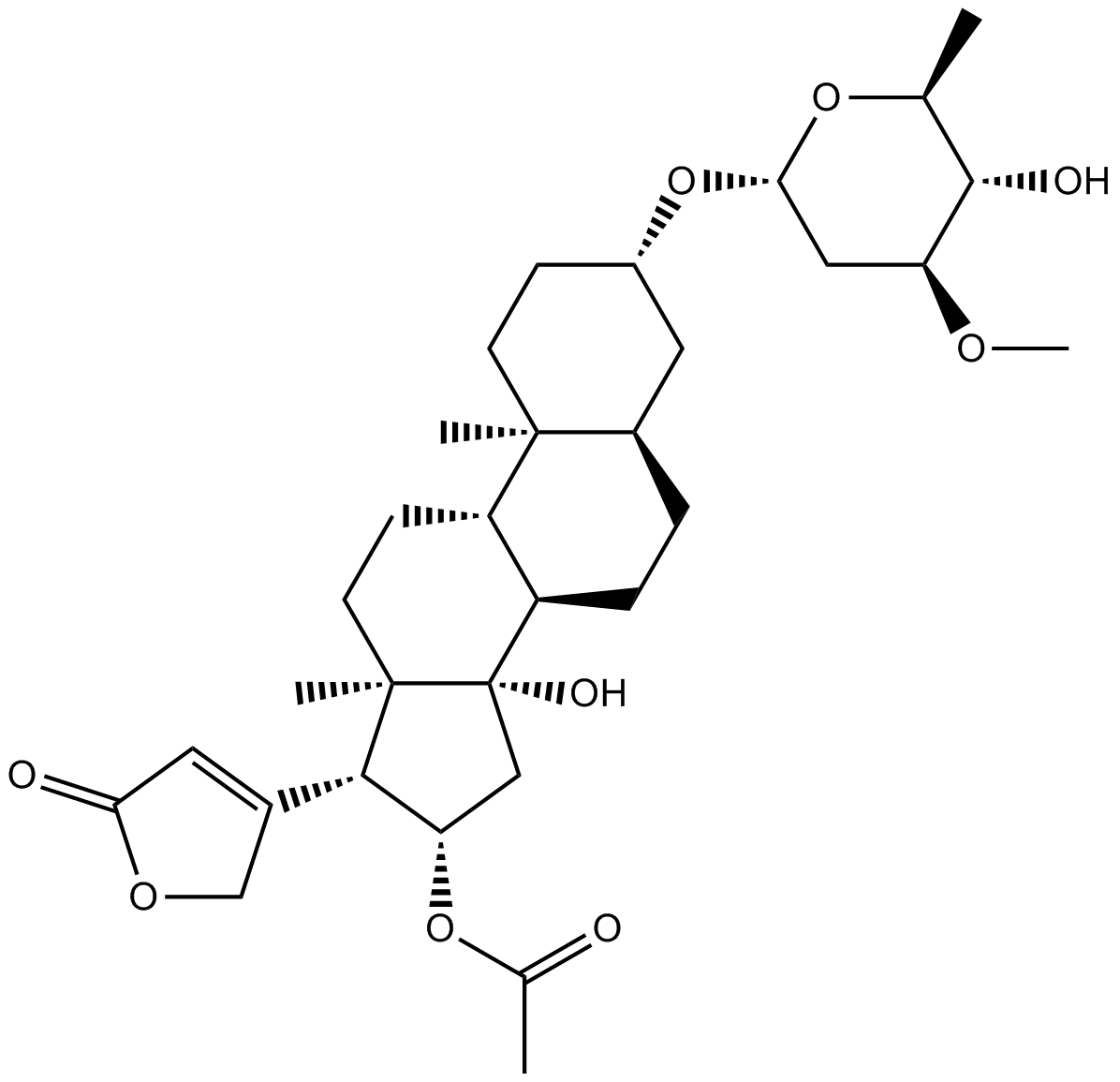
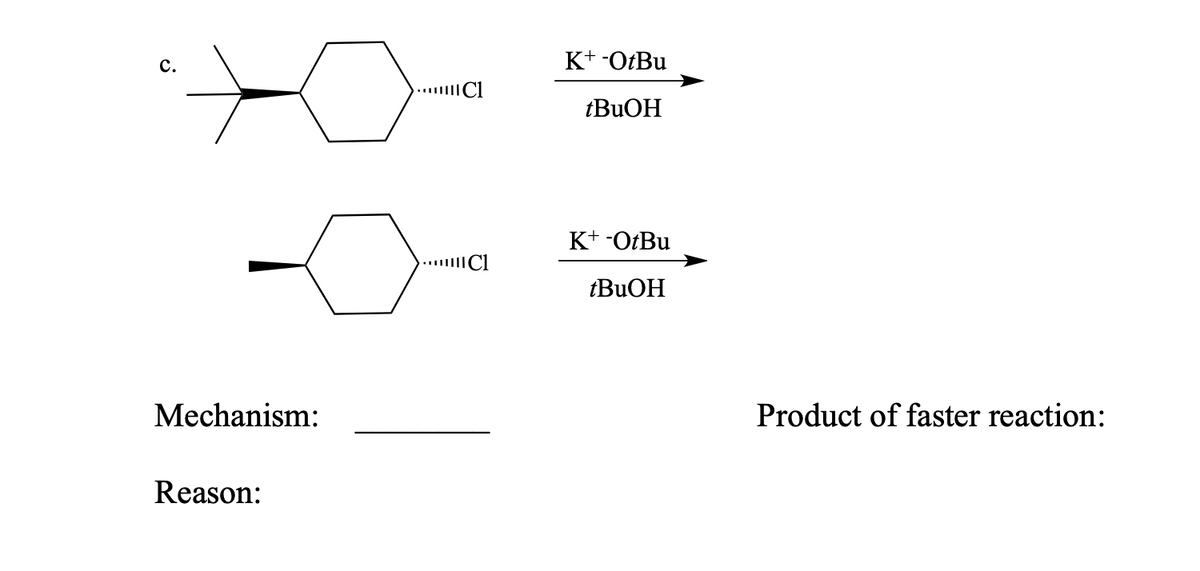
Potassium And Living Organisms
Plants, animals and humans all depend on potassium for survival and good health. The element is part of many bodily fluids and assists related functions of the human body. Most notably, potassium aids nerve functions and is found in several cell types . Plants depend on potassium for healthy growth. Potassium found in animal excretions and dead plants easily binds to clay in the soil they fall on and is thus utilized by plants. The element helps maintain osmotic pressure and cell size and plays a role in photosynthesis and energy production.
Common And Sometimes Deadly
About 2.4 percent of the mass of Earth’s crust is potassium, including billions of tons of potassium chloride, according to the Royal Society of Chemistry. It’s not surprising that humans have made use of this resource. Saltpeter was used to preserve food in the Middle Ages and is part of gunpowder, which was invented in ninth-century China. Potassium alum 2) might show up in your deodorant or in textile and leather production. Potash, a generic term for any salt containing water-soluble potassium, is a major ingredient in fertilizers.
The word “potash” comes from “pot ash,” harkening back to the original method of production of these salts. Plants are rich in potassium, so people would collect wood ash and leech out the potassium salts for use in fertilizer. In the modern day, potash is mined, with 35 million metric tons pulled from the Earth each year, according to the RSC.
Potassium is also a necessary nutrient for life as an electrolyte, it conducts electric signals in the body along with sodium, it’s crucial for proper muscle contraction. The drug potassium chloride is commonly used to treat potassium deficiency, but the dose makes the poison: Potassium chloride has also been used in lethal injections. In large enough quantities, the drug stops the heart by disrupting the electrical signals that force the muscle to contract and relax, according to a 2012 case report in the journal Case Reports in Emergency Medicine.
Ionization Energy Comparison Between K+ And Cl
So I have encountered a question which looks very suspicious to me.
If you have $\ce$ and $\ce$, Can you say that $\ce$ has more ionization energy than $\ce$?
We know for a fact that when you put more electrons it makes the the pulling force of the protons a bit scattered so the ionization energy of chlorine should go down.
The opposite happens to $\ce$ when I remove electrons the pulling force of protons is stronger than it used to be so the ionization energy goes up!
So What I am proposing here that we can’t just say that $\ce$ has more ionization energy than $\ce$ just because that happened. $\ce$ had a really high ionization energy compared to $\ce$ and the decrement of the ionization wouldn’t be that big to make it less than the ionization energy of $\ce$.
So if there is a chart or something for this that would be awesome. I hope I can get some help here.
The general reaction we are looking at when discussion ionisation enthalpy is:
$$\ce$$
With the electron removed into space . An electron is a negatively charged particle, so removing it from something negative will be easier than removing it from something positive. Thus, you have a very stable $\ce$ ion and are trying to rip away another electron from this and you need to rip it out of a core orbital all valence orbitals are already empty, the last one when you removed that previous electron to generate $\ce$ . A combination of bad and very bad gives us a very high ionisation energy for $\ce$.
You May Like: What Does Abiotic Mean In Biology
Chem 13 News University Of Waterloo Waterloo Ontario Canada
Potassium: The common theme for this tile is how biology relates to potassium. Potassium plays a critical role in the Potassium-Sodium Pump. This pump is responsible for actively transporting high concentrations of potassium ions and low amounts of sodium ions into and out of the cell. The images shown along the top and right side of the tile represent the types of foods that contain potassium in them. Foods such as brown rice, turmeric, soybeans, strawberries and etc., contain high levels of potassium which help regulate the bodys blood pressure and promote proper muscle control.
Vetura Jeyandran, co-op student assisting with the Periodic Table project, University of Waterloo, Waterloo, Ontario, Canada
K+ Energy Loss Spectrum
The energy loss spectrum of K+ ions formed in the forward direction from the collision of potassium atoms with pyrimidine at 111 eV lab frame energy, is shown in Figure 4. Note that the lowest energy loss scale appears above ~ 4 eV to account for the potassium ionization energy, 4.34 eV. Two features are visible at 10.03 and 11.91 eV, the former more intense than the latter and with a full width at half-maximum of ~1.2 eV. The position of the maxima do not shift with the collision energy within ±0.2 eV. The main anionic yield from the TOF mass spectra at all collision energies is due to CN . The energy loss E at the maximum of the features is given by E = IE EAmax, which results on an electron affinity of eV and eV, assigned to
In Figure 2 we show the three lowest calculated * MOs at 5.0 eV (
Read Also: Fsa Algebra 1 Eoc Practice Test Answers
Health Effects Of Potassium
Potassium can be found in vegetables, fruit, potatoes, meat, bread, milk and nuts. It plays an important role in the physical fluid system of humans and it assists nerve functions. Potassium, as the ion K+, concnetrate inside cells, and 95% of the body’s potassium is so located. When our kidneys are somehow malfunctioning an accumulation of potassium will consist. This can lead to disturbing heartbeats.
Potassium can effect you when breathed in. Inhalation of dust or mists can irritate the eyes, nose, throat, lungs with sneezing, coughing and sore throat. Higher exposures may cause a build up of fluid in the lungs, this can cause death. Skin and eye contact can cause severe burns leading to permanent damage.
Notable Reactions With Phosphorus
Potassium reacts so violently with water that it bursts into flame. The silvery white metal is very soft and reacts rapidly with the oxygen in air. Its chemical symbol is derived from the Latin word kalium which means “alkali”. Its English name is from potash which is the common name for a compound containing it.
| Reactant | |
|---|---|
| begins slowly at ca 200°C rapid above 300°C | KH |
| begins slowly with solid fairly rapid with liquid | K2O, K2O2, KO2 |
| extremely vigorous and frequently results in hydrogenair explosions | KOH, H2 |
| dissolves as K iron, nickel, and other metals catalyze in gas and liquid phase | KNH2 |
| molten state in liquid ammonia | K2S, K2S2, K2S4 |
| occurs readily, but is sometimes explosive | CO, C, K2CO3 |
You May Like: Algebra 1 Age Word Problems
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
What Is The Chemical Composition Of Malachite
Answer
11.5 K+
Apne doubts clear karein ab Whatsapp par bhi. Try it now.
Ab clear karein apne doubts Whatsapp par bhi. Apna phone number register karein.
Ab aap Whatsapp pe solutions paa saktey h, hum aapko message karenge.
Hurray!! Ab aap Whatsapp pe solutions paa saktey h, hum aapko ping karenge
Also Check: Who Are Paris Jackson’s Biological Parents
Potassium In The Environment
Potassium has a 2.6% abundance by mass in the earth’s crust and is found mostly in mineral form as part of feldspars and clays. Potassium easily leaches out of these minerals over time and thus has a relatively high concentration in sea water as well . Today, most of the world’s potassium is mined in Canada, the U.S., and Chile but was originally monopolized by Germany.
Detection By Taste Buds
Potassium can be detected by taste because it triggers three of the five types of taste sensations, according to concentration. Dilute solutions of potassium ions taste sweet, allowing moderate concentrations in milk and juices, while higher concentrations become increasingly bitter/alkaline, and finally also salty to the taste. The combined bitterness and saltiness of high-potassium solutions makes high-dose potassium supplementation by liquid drinks a palatability challenge.
Don’t Miss: Exponential Growth And Decay Algebra 1 Worksheet
The Naked Truth About K+ Selectivity
volume 10, pages 799800
-
2287 Accesses
Potassium channels rapidly move K+ ions across cell membranes while blocking Na+, but how these two effects are achieved simultaneously has remained unclear. Now, extensive molecular simulations show a single mechanism that features fully dehydrated ions can explain both rapid transport and impeccable selectivity.
What Is The Concentration Of Ions That Remain
A solution of 120 mL of 0.200 M KOH is mixed with a solution of 290 mL of 0.210 NiSO4
What concentration of SO4 and K ions remain?
My last problem…erg. HelP?
At the beginning of the exp, there are:0.024 moles of KOH
0.0489 moles of NiSO4 in excess.
I don’t know what to do from here
Submitted by kingchemist on Wed, 10/13/2010 – 05:28
At the beginning of the exp, there are:0.024 moles of KOH 0.0609 moles of NiSO4 (starting with 0.0609 moles SO4
KOH is limiting, so..0.0489 moles of NiSO4 in excess. So there will be 0.0489 moles of SO4 ions unused
If 0.024 moles KOH reacted, then 0.012 mole of K2SO4 will be made and contain 0.012 mole SO4 ionsTotal SO4 ions = 0.0489 + 0.012 = 0.609 mole of SO4 (which has to be the same as the starting number of moles of SO4
0.024 moles KOH reacted, then 0.012 mole of K2SO4 will be made and contain 0.024 mole K+ ions
The reaction is a precipitation between Ni2+ and OH- ions, so K+ and SO4 are spectating. This means the number of these ions at the start in the solutions will be the same in the mixture at the end.
2K+OH- +Ni2+SO42- –> 2SO42- + Ni2+2
2K+ + 2OH- +Ni2+ + SO42- –> 2K+ + SO42- + Ni2+2
2OH- +Ni2+ –> Ni2+2
You May Like: Kendall Hunt Geometry Answer Key
Students Are Also Searching For
- 4.what is the area of the square below
- which major characteristic of romantic art of the nineteenth century does this painting share?
- volcanologists and seismologists are specialized ______.
If you have more homework to do you can use the search bar to find the answer to other homework: 900 have done it today and 156 in the last hour.
Help your mates do their homework and share Top Homework Answers with them, its completely free and easy to use!
Contents
What Is The Name Simbolize For K+ Ion
-
About saying good job to the person you congratulate.
- Réponse publiée par: 09330399672
be a symbol of something
be considered a typical or perfect example.
- Réponse publiée par: maledabacuetes
Explanation:
noun. the act or process of symbolizing. Psychoanalysis. an unconscious mental process whereby one object or idea comes to stand for another through some part, quality, or aspect that the two share in common, with the symbol carrying the emotional feelings vested in the initial object or idea.
- Réponse publiée par: kenn14
expressive of sympathetic pleasure or joy on account of someone’s success or good fortune.
- Réponse publiée par: shannel99
I drew this. It represents me and my understanding of life. It’s my acknowledgement to myself of the light that is ever present within me, and the darkness that sometimes blocks the light from shining. I am all heart underneath everything, and I use my hands to heal. The journey has been a real doosey for me, but I’m stoked to be where I am and love the story so far. It is magical, full of twists and turns but underneath everything that has been, love is the essence that carries me, and is the underlying essence of life.
- Réponse publiée par: kuanjunjunkuan
Symbol po yan sanna makatulong
- Réponse publiée par: elaineeee
um…so where is the picture
-
Upang kumatawan, ipahayag, o makilala sa pamamagitan ng isang simbolo.
Explanation:
Recommended Reading: G Meaning In Physics
What Is The Correct Order Of Decreasing Size Of The Following Ions Multiple Choice Ca2+ > K+ > Cl > P3 K+ > Cl > Ca2+ > P3 P3 > Cl > K+ > Ca2+ Correct K+ > Cl > P3 > Ca2+ None Of These Choices Are Correct
Answers
Explanation:Isoelectronic specie are defined as the molecules which have the same number of electrons.Atomic number of calcium is 20 and thus has 20 electrons. is formed by loss of 2 electrons and thus has 18 electrons.Atomic number of potassium is 19 and thus has 19 electrons. is formed by loss of 1 electron and thus has 18 electrons.Atomic number of chlorine is 17 and thus has 17 electrons. is formed by gain of 1 electron and thus has 18 electrons.Atomic number of phosphorous is 15 and thus has 13 electrons. is formed by gain of 3 electrons and thus has 18 electrons.As the number of electrons are same , the more is the nuclear charge i.e. the number of protons , more will the force of attraction towards the nuclei and thus smaller will be the radii and vice versa.Thus, order of decreasing ionic radii for the given ions is as follows. >
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Read Also: What Does Mole Mean In Chemistry
What Is The Big O Notation
Big O notation is a mathematical notation that describes the limiting behavior of a function when the argument tends towards a particular value or infinity. In computer science, big O notation is used to classify algorithms according to how their run time or space requirements grow as the input size grows.
Cosmic Formation And Distribution
Potassium is formed in supernovae by nucleosynthesis from lighter atoms. Potassium is principally created in Type II supernovae via an explosive oxygen-burning process.40K is also formed in s-process nucleosynthesis and the neon burning process.
Potassium is the 20th most abundant element in the solar system and the 17th most abundant element by weight in the Earth. It makes up about 2.6% of the weight of the earth’s crust and is the seventh most abundant element in the crust. The potassium concentration in seawater is 0.39 g/L , about one twenty-seventh the concentration of sodium.
Recommended Reading: Unit 1 Test Geometry Basics Answer Key
Who Knew
- Low potassium in the body is called hypokalemia. Symptoms include muscle cramps, weakness and irregular heartbeat, according to the University of Maryland Medical Center. High potassium, called hyperkalemia, causes similar symptoms.
- Your potassium levels may be off without these extreme symptoms, however. A 2008 study published in the journal Hypertension found that certain blood pressure drugs can result in low potassium levels, which in turn raises the risk of Type 2 diabetes.
- Sounds fun: According to Humphry Davy’s lab assistant, the scientist reacted with glee when he discovered his new element. “I have been told by Mr. Edmund Davy, his relative and assistant that when he saw the minute globules of potassium burst through the crust of potash, and take fire as they entered the atmosphere could not contain his joy he actually danced about the room in ecstatic delight,” John Davy wrote in his “Memoirs of the life of Sir Humphry Davy.”