Differences Between The Inductive Effect And The Resonance Effect
First, what is the inductive effect ? And what is the resonance effect ? Lets think of them both as effects caused by the functional groups attached to the molecule. Depending on what substituents are bonded to the molecule, there will be many differences, such as different acidity.
The differences between the two are as follows.
- Inductive effect: effect of -bond
- Resonance effect: Effect of -bond
Electronic orbitals include s and p orbitals, and these orbitals form bonds. Among the bonds formed by these s and p orbitals, the single bond is the -bond. The substituent effect of the -bond is the inductive effect.
On the other hand, some molecules form double or triple bonds. The part of the molecule that forms a double or triple bond is called a bond if a pi bond is present, you can write resonance structures. The resonance effect is the result of resonance by the substituent, which changes the orientation and the acidity of the molecule.
Roughly speaking, understand that the inductive effect affects the alkyl chain, and the resonance effect affects the benzene ring.
Acidic Strength Of Carboxylic Acids And Phenols:
A protonic acid is always in equilibrium with its conjugate base that is formed by loss of a proton. Any factor that stabilizes the negatively charged conjugate base favors greater ionization of acid. i.e increases the acidic strength.
The electron withdrawing groups showing negative inductive effect reduce the negative charge on the conjugate base by pulling the electron density and thus by stabilizing it. Hence the acidic strength increases when -I groups are present.
On the contrary, the +I groups decrease the acidic strength as they destabilize the conjugate base of acid by releasing electron density which in turn increases the negative charge on the anion and thus destabilizing it.
E.g.
i) The acidic strength increases with increase in the number of electron withdrawing Fluorine atoms as shown below.
CH3COOH < CH2FCOOH < CHF2COOH < CF3COOH
ii) Formic acid is stronger acid than acetic acid since the CH3group destabilizes the carboxylate ion.
On the same lines, the acidic strength of phenols increases when -I groups are present on the ring.
E.g. The p-nitrophenol is stronger acid than phenol since the -NO2group is a -I group that withdraws electron density. Whereas, the para-cresol is weaker acid than phenol, since the -CH3group shows positive inductive effect .
For example, the order of acidic strength following phenols is:
Thus the order of basic strength of alkyl and aryl amines with respect to ammonia is :CH3NH2> NH3> C6H5NH2
Resonance Effect Or Mesomeric Effect
The electron withdrawing or releasing effect attributed to a substituent through delocalization of p or electrons, which can be visualized by drawing various canonical forms, is known as mesomeric effect or resonance effect. It is symbolized by M or R.
Negative resonance or mesomeric effect :It is shown by substituents or groups that withdraw electrons by delocalization mechanism from rest of the molecule and are denoted by -M or -R. The electron density on rest of the molecular entity is decreased due to this effect.
E.g. -NO2, Carbony group , -CN, -COOH, -SO3H etc.
Positive resonance or mesomeric effect : The groups show positive mesomeric effect when they release electrons to the rest of the molecule by delocalization. These groups are denoted by +M or +R. Due to this effect, the electron density on rest of the molecular entity is increased.
E.g. -OH, -OR, -SH, -SR, -NH2, -NR2 etc.
1) The negative resonance effect of carbonyl group is shown below. It withdraws electrons by delocalization of electrons and reduces the electron density particularly on 3rd carbon.
2) The negative mesomeric effect shown by cyanide group in acrylonitrile is illustrated below. The electron density on third carbon decreases due to delocalization of electrons towards cyanide group.
Because of negative resonance effect, the above compounds act as good .
This is the reason for why nitro group deactivates the benzene ring towards electrophilic substitution reaction.
Also Check: Nc In Physics
What Is Resonance Effect
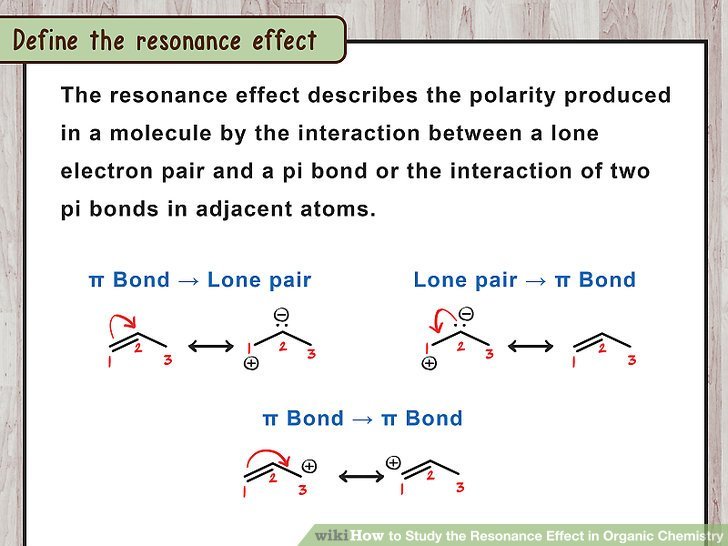
Resonance effect describes the effect on the stability of a molecule due to the interaction between pi bond electrons. Lone electron pairs can also contribute to the resonance of a molecule if there are any lone pairs present on atoms of the molecule.
The resonance effect causes the delocalization of electrons between atoms. Molecules having double bonds are involved in resonance. In order to determine the real structure of a molecule, we can use resonance structures. The real structure of a molecule is an intermediate structure obtained through resonance stabilization. Resonance structures are not isomers of the original molecule.
Figure 2: Resonance Effect in Nitrobenzene
If a particular molecule has no other resonance structures but only one structure, then it is the most stable structure in which the molecule can exist. Resonance structures are drawn as Lewis structures. By writing all possible structures for a molecule, we can determine the most stable intermediate structure for that molecule.
Rules For Writing Resonance Structures
Recommended Reading: What Does Amu
Delocalization And Resonance Structures Rules
In resonance structures, the electrons are able to move to help stabilize the molecule. This movement of the electrons is called delocalization.
What Is Inductive Effect
Inductive effect is the effect that is caused by the transmission of an electrical charge throughout a chain of atoms. This charge transmission will finally result in a fixed electrical charge on atoms. The inductive effect occurs due to the differences in the electronegative values of atoms of a molecule.
An atom with a higher electronegativity tends to attract electrons towards themselves than lower electronegative atoms do. Therefore, when a highly electronegative atom and a low electronegative atom are in a covalent bond, the bond electrons are attracted towards the highly electronegative atom. This induces the low electronegative atom to get a partially positive charge. The highly electronegative atom will get a partial negative charge. This is called bond polarization.
The inductive effect is found in two ways as follows.
Read Also: Holt Geometry Answers
Difference Between Resonance And Inductive Effect
- The polarization of one bond caused by the polarization of an adjacent bond is known as an inductive effect. The phenomena in which two or more structures can be written for true structure of a molecule but none of them alone can explain all the properties of the molecule is called Resonance Effcet.
- The electronegativity difference between the two atoms in the bond directly affects on the inductive effect while Number of Resonating structure effect the stability.
How To Distinguish Between Electron
How do we distinguish between the electron-donating and the electron-withdrawing group on the benzene ring? To do this, look for double bonds in the substituents.
For example, the following substituents are involved as electron-donating groups on the benzene ring.
- Methoxy group
- Hydroxy group
- Amino group
If we focus on the atoms directly bonded to the benzene ring , we find that they are all single bonds. As a result, these substituents are electron-donating groups on the benzene ring.
On the other hand, what if there is a double bond in the substituent? In this case, they act as electron-withdrawing groups.
- Carbonyl group
- Nitro group
- Cyano group
The presence of a double bond allows electrons to be drawn into the substituent group by resonance. As a result, it becomes an electron-withdrawing group.
When an oxygen or nitrogen atom is bonded to the substituent, it becomes an electron-donating group if the substituent has only single bonds. On the other hand, when a substituent that has a double bond is bonded, it becomes an electron-withdrawing group. Although there are exceptions, this is a rough understanding.
Read Also: Chapter 7 Test Algebra 1 Answer Key
Example Of Inductive Effect
The C-Cl bond in the butyl chloride, CH3-CH2-CH2-CH2-Cl is polarized due to electronegativity difference. The electrons are withdrawn by the chlorine atom. Thus the first carbon atom gets partial positive charge. In turn, this carbon atom drags electron density partially from the next carbon, which also gets partial positive charge. This will continue further and is how the inductive effect is transmitted through the carbon chain.
One must note that the inductive effect weakens away along the chain and is not that much significant beyond the 3rd carbon atom.
Also note that inductive effect is a permanent effect and is inherent to the molecule, while the electromeric effect is a temporary effect is only created in the presence of attacking electrophilic or nucleophilic reagents.
Quantum Mechanical Description In Valence Bond Theory
Resonance has a deeper significance in the mathematical formalism of valence bond theory . Quantum mechanics requires that the wavefunction of a molecule obey its observed symmetry. If a single contributing structure does not achieve this, resonance is invoked.
For example, in benzene, valence bond theory begins with the two Kekulé structures which do not individually possess the sixfold symmetry of the real molecule. The theory constructs the actual wave function as a linear superposition of the wave functions representing the two structures. As both Kekulé structures have equal energy, they are equal contributors to the overall structure the superposition is an equally weighted average, or a 1:1 linear combination of the two in the case of benzene. The symmetric combination gives the ground state, while the antisymmetric combination gives the first excited state, as shown.
In general, the superposition is written with undetermined coefficients, which are then variationallyoptimized to find the lowest possible energy for the given set of basis wave functions. When more contributing structures are included, the molecular wave function becomes more accurate and more excited states can be derived from different combinations of the contributing structures.
Recommended Reading: Define Movement Geography
What Is A Resonance In Chemistry
resonancestructure
Regarding this, what is an example of a resonance?
example of resonanceresonant
What are the rules for drawing resonance structures?
Rules to remember for recognising resonance structures:
- Atoms never move.
- You can only move electrons in bonds or lone pairs
- The overall charge of the system must remain the same.
- The bonding framework of a molecule must remain intact.
Resonance Effects Take Into Account The Delocalization Of Electrons
The inductive effect in alkyl chains is simple. The inductive effect is the lowering of the electron density due to the bonding of functional groups with a high degree of electronegativity. This results in a higher degree of acidity .
The resonance effect , on the other hand, is a bit more complicated. In contrast to the inductive effect, which only requires considering the effects of single bonds, the resonance effect requires considering the effects of double and triple bonds. The effect of the -bond is the resonance effect.
Among these double bonds, the resonance effect has a strong influence on the orientation and acidity of aromatic compounds . It is also responsible for the reaction rates of organic chemical reactions.
For compounds with conjugated structures, the resonance effect should be taken into account. Roughly speaking, we can think of resonance effects as those related to the reactivity of the benzene ring.
Recommended Reading: Holt Geometry Chapter 2 Test
The Resonance Effect Is Stronger Than The Inductive Effect
Oxygen and nitrogen atoms are typical examples of electron-withdrawing groups. So when hydroxy groups , methoxy groups and amino groups are attached to the alkyl chain, they become electron-withdrawing groups. Any of these substituents can be considered to be an electron-withdrawing group.
In aromatic compounds, on the other hand, the situation is different. Sometimes the substituent becomes an electron-donating group, and sometimes it becomes an electron-withdrawing group. This is because they resonate.
The more you can write resonance structures, the more stable the compound is. If there is resonance, the electrons can move to many places with it. This is called the delocalization of electrons. The greater the degree of delocalization, the more stable the electron state becomes.
In order to draw resonance structures, it is essential that the compound has a double bond. Since the benzene ring has double bonds, we can draw resonance structures for any aromatic ring compound. For example, here is the resonance of aniline.
If we focus on the nitrogen atom of aniline, we can see that electrons are pushed out from the nitrogen atom toward the benzene ring. In other words, the nitrogen of aniline acts as an electron-donating group.
Stability Of Carbocations :
The stability of carbocations increase when +I groups like alkyl groups are present adjacent to positively charged carbon. The +I groups reduce the positive charge on the carbon by donating negative charge density through positive inductive effect. This results in greater stability of carbocation.
Whereas, the -I groups destabilize the carbocations as they increase the positive charge by withdrawing electron density.
Note that any factor that increases the charge on an ion results in destabilization while any factor that reduces the charge results in stabilization of that ion.
For example, the order of stability of a few carbocations containing alkyl groups is as follows:
The tertiary carbocation containing three alkyl groups is more stable than the secondary carbocation with two alkyl groups and which in turn is more stable than the primary carbocation. Methyl carbocation is the least stable among the given.
In the same way the stability of free radicals increases with increase in the number of alkyl groups.
Thus the stability of different free radicals is:
Don’t Miss: Chapter 2 Test Form 2b
Mesomeric Effect And Its Strength
While solving a question in organic chemistry, I needed to compare the strength of mesomeric effects of various groups. I looked it up on Wikipedia, and it goes like this :
+M EFFECT ORDER :
O > NH2 > NHR > OR > NHCOR > OCOR > Ph > F > Cl > Br > I
-M EFFECT ORDER :
NO2 > CN > –S2OH > CHO > C=O > COOCOR > COOR > COOH > CONH2 > COO
What is the way to understand such comparisons ? . Why does the -OH group show stronger mesomeric effect than the -NH2 group ? Also, why is an -OR group weaker than the -NH2 group ? Can somebody please explain I was unable to find a reasonable explanation through a google search.
Types Of Resonance Effects
There are two types of Resonance effects namely positive resonance effect and negative resonance effect.
Also Check: Algebra 2 Regents 2016
The Group Leader $\ce$ :
First,let us look at some enthalpy values:
$\ce$
$\ce$
As you can clearly see, adding an electron to $\ce$ is an endothermic process, which means energy will have to be provided externally for pushing more electron density inside $\ce$.
That is pretty obvious as well, because you are forcing an electron into an already negative ion. It’s not going to go in willingly! The second electron affinity of oxygen is particularly high because the electron is being forced into a small, very electron-dense space.
Conversely, this means that the reverse reaction for will be pretty favorable, in the sense that $\ce$ will quite readily give away electron density to a suitable acceptor atom,as is evident from the enthalpy values. Hence, $\ce$ leads the charge in terms of +M effect
It is to be kept in mind that the factor of compatibility of overlap still holds. Donation from $\ce$ to, say, $\ce$ will still not be as effective as that to $\ce$
Resonance Structures In Organic Chemistry
Resonance stabilization effect , as briefly mentioned in Section 1.3, is one of the fundamental concepts of Organic Chemistry and has broad applications. The discussion of resonance effect heavily relies on the understanding of resonance structures. Here we will focus on how to draw resonance structures for organic chemistry species, and how to compare the relative stabilities between the structures.
According to resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the species is. Therefore, to predict whether the resonance effect applies or not, we usually need to construct new resonance structures based on the original one that is available. There are some very important rules we need to follow for such purposes.
Guidelines for Drawing Resonance Structures:
- All resonance structures must be valid Lewis structures.
- All resonance structures must have the same atom connectivity, and only differ in the electron arrangement.
- All resonance structures have the same number of electrons and net charge.
- To move electrons, only electrons and lone-pair electrons can be moved from the higher electron density area to lower electron density area by following one of the three transformations:
- bond forms another bond
- bond forms the lone pair electrons
- lone pair electrons forms a bond.
arrow pushing
Also Check: Hawkes Learning College Algebra Answers