How Do Common And Uncommon Ions Affect Ksp
For a given equilibrium, a reaction with a common ion present has a lower Ksp , and the reaction without the ion has a greater Ksp. Salt Effect : Having an opposing effect on the Ksp value compared to the common ion effect, uncommon ions increase the Ksp value. Uncommon ions are ions other than those involved in equilibrium.
What Does Ksp Mean
Also to know is, what does the KSP tell you?
Ksp is the equilibrium between a solid and its respective ions in a solution. The value of the constant identifies the degree of which the compound can dissociate in water. For example the higher the Ksp the more soluble the compound is.
Likewise, what KSP is considered soluble? Explanation: K sp is often written in scientific notation like 2.5 x 103. The larger the negative exponent the less soluble the compound is in solution. The larger the real value of the Ksp the more soluble the compound is in solution 2.5 x 103 > 2.5 x 106.
Similarly, you may ask, what is meant by solubility product?
Solubility Product DefinitionD. Updated January 12, 2019. A solubility product, or Ksp, is the equilibrium constant for a chemical reaction in which a solid ionic compound dissolves to yield its ions in solution. Also Known As: Ksp, ion product, solubility product constant.
What factors affect KSP?
Solubility is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature. There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
Acid Dissociation Constant From Ph
The acid dissociation constant may be found it the pH is known. For example:
Calculate the acid dissociation constant Ka for a 0.2 M aqueous solution of propionic acid that is found to have a pH value of 4.88.
To solve the problem, first, write the chemical equation for the reaction. You should be able to recognize propionic acid is a weak acid . It’s dissociation in water is:
CH3CH2CO2H + H2 H3O++ CH3CH2CO2-
Set up a table to keep track of the initial conditions, change in conditions, and equilibrium concentration of the species. This is sometimes called an ICE table:
Also Check: Kuta Software Simplifying Radical Expressions Answers
Solubility Product Constant Table
Below is a chart showing the $K_s_p$ values for many common substances. The $K_s_p$ values are for when the substances are around 25 degrees Celsius, which is standard. Because the $K_s_p$ values are so small, there may be minor differences in their values depending on which source you use. The data in this chart comes from the University of Rhode Islands Department of Chemistry.
| Substance |
What Is Q And K
Q can be used to determine which direction a reaction will shift to reach equilibrium. If K > Q, a reaction will proceed forward, converting reactants into products. If K < Q, the reaction will proceed in the reverse direction, converting products into reactants. If Q = K then the system is already at equilibrium.
Read Also: New England Colony Climate
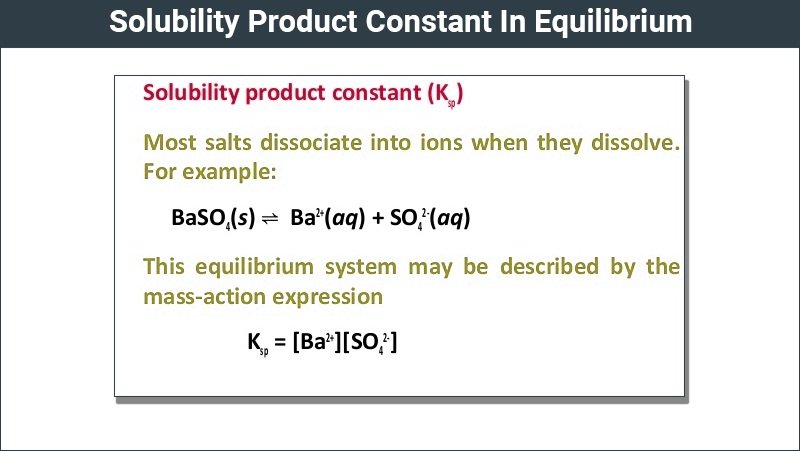
What Is Solubility Product Constant
The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp value it has. Consider the general dissolution reaction below :
Solubility As An Equilibrium Process
So let’s imgaine an experiment in which I have 100 mL water at 25°C and I add solid barium sulfate 0.0002 g at a time, stirring between each addition and analysing the solution to determine the concentration of barium ions ) and sulfate ions .
The results of the experiment might look like this:
| total mass BaSO4 |
|---|
Don’t Miss: Geometry Basics Segment Addition Postulate Worksheet Answer Key
What Is Solubility Product Ksp
The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. It is denoted by the symbol Ksp.
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
Solubility is defined as a property of a substance called solute to get dissolved in a solvent in order to form a solution.The solubility of ionic compounds in water varies to a great deal. Some compounds are highly soluble and may even absorb moisture from the atmosphere whereas others are highly insoluble.
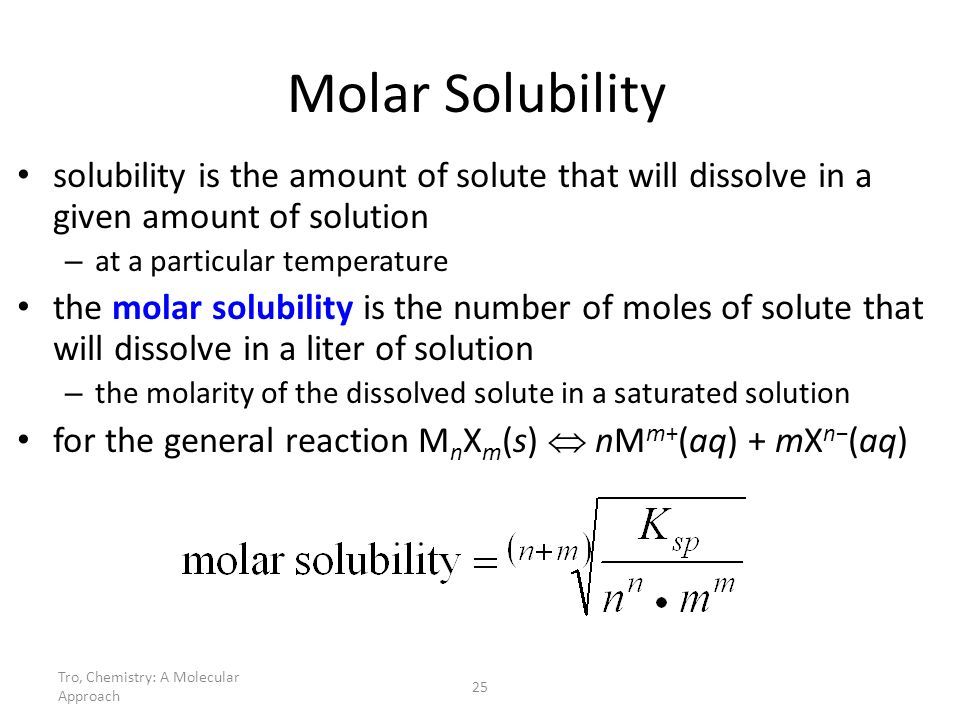
Solving For $k: S: P$ With Solubility
In order to calculate a value for $K_s_p$, you need to have molar solubility values or be able to find them.
Question: Determine the $K_s_p$ of AgBr , given that its molar solubility is 5.71 x $10^^7$ moles per liter.
First, we need to write out the two equations.
AgBr $Ag^$ + $Br^$
$K_s_p$ =
Now, since in this problem we’re solving for an actual value of $K_s_p$, we plug in the solubility values we were given:
$K_s_p$ = = 3.26 x $10^^13$
The value of $K_s_p$ is 3.26 x $10^^13$
Don’t Miss: Geopolitics Definition Ap Human Geography
Understanding Kb And Pkb
Kb is the base dissociation constant. The base dissociation constant is a measure of how completely a base dissociates into its component ions in water.
- Kb = /
- pKb = -log Kb
A large Kb value indicates the high level of dissociation of a strong base. A lower pKb value indicates a stronger base.
pKa and pKb are related by the simple relation:
- pKa + pKb = 14
Writing $k: S: P$ Expressions
Below is the solubility product equation which is followed by four $K_s_p$ chemistry problems so you can see how to write out $K_s_p$ expressions.
For the reaction $A_aB_b$ $aA^b^$ + $bB^a^$
The solubility expression is $K_s_p$= $^a$ $^b$
The first equation is known as a dissociation equation, and the second is the balanced $K_s_p$ expression.
For these equations:
- A and B represent different ions and solids. In these equations, they are also referred to as “products”.
- a and b represent coefficients used to balance the equation
- and indicate which state the product is in
- Brackets stand for molar concentration. So represents the molar concentration of AgCl.
In order to write $K_s_p$ expressions correctly, you need to have a good knowledge of chemical names, polyatomic ions, and the charges associated with each ion. Also, the key thing to be aware of with these equations is that each concentration is raised to the power of its coefficient in the balanced $K_s_p$ expression.
Lets look at a few examples.
$PbBr_2$ $Pb^2^$ + $2Br^$
$K_s_p$= $$ $^2$
In this problem, dont forget to square the Br in the $K_s_p$ equation. You do this because of the coefficient 2 in the dissociation equation.
CuS $Cu^$ + S¯
$K_s_p$=
$Ag_2CrO_4$ 2$Ag^$ + $CrO_4^2^$
$K_s_p$= $^2$
$Cu_3$ $^2$ $3Cu^2^$ + $2PO_4^3^$
$K_s_p$ = $^3$ $^2$
Don’t Miss: Core Connections Algebra Chapter 1 Answers
To Predict If A Precipitate Will Form In Reactions
When we know the $K_s_p$ value of a solute, we can figure out if a precipitate will occur if a solution of its ions is mixed. Below are the two rules that determine the formation of a precipitate.
- Ionic product > $K_s_p$ then precipitation will occur
- Ionic product < $K_s_p$ then precipitation will not occur
Understanding Ka And Pka
Ka, pKa, Kb, and pKb are most helpful when predicting whether a species will donate or accept protons at a specific pH value. They describe the degree of ionization of an acid or base and are true indicators of acid or base strength because adding water to a solution will not change the equilibrium constant. Ka and pKa relate to acids, while Kb and pKb deal with bases. Like pH and pOH, these values also account for hydrogen ion or proton concentration or hydroxide ion concentration .
Ka and Kb are related to each other through the ion constant for water, Kw:
- Kw = Ka x Kb
Ka is the acid dissociation constant. pKa is simply the -log of this constant. Similarly, Kb is the base dissociation constant, while pKb is the -log of the constant. The acid and base dissociation constants are usually expressed in terms of moles per liter . Acids and bases dissociate according to general equations:
- HA + H2O â A- + H3O+
In the formulas, A stands for acid and B for base.
- Ka = /
- pKa = – log Ka
- at half the equivalence point, pH = pKa = -log Ka
A large Ka value indicates a strong acid because it means the acid is largely dissociated into its ions. A large Ka value also means the formation of products in the reaction is favored. A small Ka value means little of the acid dissociates, so you have a weak acid. The Ka value for most weak acids ranges from 10-2 to 10-14.
Don’t Miss: Paris Jackson Father Biological
How Do You Calculate $k: S: P$
In this section, we explain how to write out $K_s_p$ chemistry expressions and how to solve for the value of $K_s_p$. For most chemistry classes, youll rarely need to solve for the value of $K_s_p$ most of the time youll be writing out the expressions or using $K_s_p$ values to solve for solubility .
Definition Of Solubility Product Ksp
The solubility product, Ksp, applies in situations where salts do not fully dissolve in a solvent. The solvent is generally water.A substance’s solubility product is the mathematical product of its dissolved ion concentrations raised to the power of their stoichiometric coefficients.This definition can be a little hard to understand on first reading. It’s easier to see how things work with examples, as you’ll see below.
Recommended Reading: Who Are The Biological Parents Of Prince Paris And Blanket
Key Difference Ksp Vs Qsp
Ksp is the solubility product constant and Qsp is the solubility product quotient. The key difference between Ksp and Qsp is that Ksp indicates the solubility of a substance whereas Qsp indicates the current state of a solution. The solubility product is the product of concentrations of ionic species present in a solution when a substance is dissolved in a solvent such as water.
The solubility product is determined when the solution is saturated with that substance. Solubility product quotient is the product of concentrations of ionic species in a solution at any time before the saturation or after the solution is saturated. It is sometimes known as the ionic product.
Ksp Chemistry: Complete Guide To The Solubility Constant
Are you learning chemistry but dont quite understand the solubility product constant or want to learn more about it? Not sure how to calculate molar solubility from $K_s_p$? The solubility constant, or $K_s_p$, is an important part of chemistry, particularly when youre working with solubility equations or analyzing the solubility of different solutes. When you have a solid grasp of $K_s_p$, those questions become much easier to answer!
In this $K_s_p$ chemistry guide, well explain the $K_s_p$ chemistry definition, how to solve for it , which factors affect it, and why its important. At the bottom of this guide, we also have a table with the $K_s_p$ values for a long list of substances to make it easy for you to find solubility constant values.
Recommended Reading: Core Connections Algebra 1 Chapter 8 Answers
Solubility Of Ionic Compounds
- The solubility of an ionic compound is usually expressed as the amount of solute dissolved per volume of solvent. This can be either expressed as mass per volume e.g. g/100 mL or as molarity, mol L-1 .
- When solubility of an ionic compound is known, its Ksp can be determined. For example, at 25ºC, the solubility of PbF2 is found to be 0.64 g/L. Calculate the Ksp of PbF2.
|
Step 1: Convert solubility into mol L-1 by dividing by the molar mass of the ionic compound. In this case, the molar mass of PbF2 = 245.2 g mol-1 |
Question 1
Lead sulfate is a key component in lead acid car batteries.
Its solubility in water at 25°C is 4.25 `xx` 10-3 g/100 mL. What is the Ksp of PbSO4?
In terms of their constituent ions, explain why lead fluoride has a much greater solubility than lead sulfate.
Question 2
The solubility of silver chloride in water at 25ºC is 1.34 `xx` 10-5.What is the Kspof silver chloride?
Question 3
The solubility of calcium hydroxide in water at 25ºC is 0.074 g/100 mL. What is the Kspof calcium hydroxide?
Determining solubility from Ksp
Question 1
Kspof barium sulfate is 11.1 `xx` 10-10. What is the solubility of barium sulfate in moles per litre? What about in grams per mL?
Question 2
Kspof iron hydroxide is 14.4 `xx` 10-38. What is the solubility of iron hydroxide in moles per litre? What about in grams per mL?
Relationship Between Solubility And Ksp
- General Biology at OpenStax CNX
Learning Objectives
- Quantitatively related \ to solubility
Considering the relation between solubility and \ is important when describing the solubility of slightly ionic compounds. However, this article discusses ionic compounds that are difficult to dissolve they are considered “slightly soluble” or “almost insoluble.” Solubility product constants ) are given to those solutes, and these constants can be used to find the molar solubility of the compounds that make the solute. This relationship also facilitates finding the \ of a slightly soluble solute from its solubility.
Also Check: Prince Jackson Biological Father
What Is The Difference Between Solubility And Solubility Product Constant
The solubility of a substance in a solvent is the total amount of the solute that can be dissolved in the solvent at equilibrium. On the other hand, the solubility product constant is an equilibrium constant that provides insight into the equilibrium between the solid solute and its constituent ions that are dissociated across the solution.
What Does The Keq Tell You
4.2/5Keqtells youKeqyouin-depth answer
If Keq is much greater than 1 , then the position of equilibrium is to the right more products are present at equilibrium. If Keq = 1, then the position of equilibrium is in the center, the amount of products is roughly equal to the amount of reactants at equilibrium.
what are the effects of KEQ? The value of Keq will not change in any case except for when there is a change in temperature. c) a change in volume a decrease in volume causes an increase in pressure. To reduce the pressure, the system will try to reduce the number of gas molecules and the reaction will shift to the side with fewer gas molecules.
Accordingly, what does the equilibrium constant tell you?
The magnitude of the equilibrium constant, K, indicates the extent to which a reaction will proceed: If K is a large number, it means that the equilibrium concentration of the products is large. If K is a small number, it means that the equilibrium concentration of the reactants is large.
What does it mean if KEQ is greater than 1 answers com?
If a reaction creates far more products than reactants, the numerator is large and the denominator is small. That means Keq will be large. So when Keq is large, equilibrium is to the far right of the reaction. If few products form, and many reactants remain, the numerator is small and the denominator is large.
Recommended Reading: Define Y Intercept In Math
What Does It Mean If Keq 1
4.8/5
Keeping this in consideration, what does the value of KEQ tell you?
If Keq is very large, the concentration of the products is much greater than the concentration of the reactants. If Keq is very small, the concentration of the reactants is much greater than the concentration of the products.
Similarly, what is the relationship between KEQ and Delta G? A non-spontaneous reaction has a positive delta G and a small K value. When delta G is equal to zero and K is around one, the reaction is at equilibrium. You have learned the relationship linking these two properties. This relationship allows us to relate the standard free energy change to the equilibrium constant.
Additionally, what does KEQ stand for?
Equilibrium constant
What does KSP mean?
Solubility product constant is simplified equilibrium constant defined for equilibrium between a solids and its respective ions in a solution. Its value indicates the degree to which a compound dissociates in water. The higher the solubility product constant, the more soluble the compound.
A system in equilibrium is affected by the following factors:
- Change of concentration of any reactant or product.
- Change of temperature of the system.
- Change of pressure of the system.
- Addition of catalyst.
What Does The P Mean
Whenever you see a “p” in front of a value, like pH, pKa, and pKb, it means you’re dealing with a -log of the value following the “p”. For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.
Don’t Miss: What Does Abiotic Factors Mean
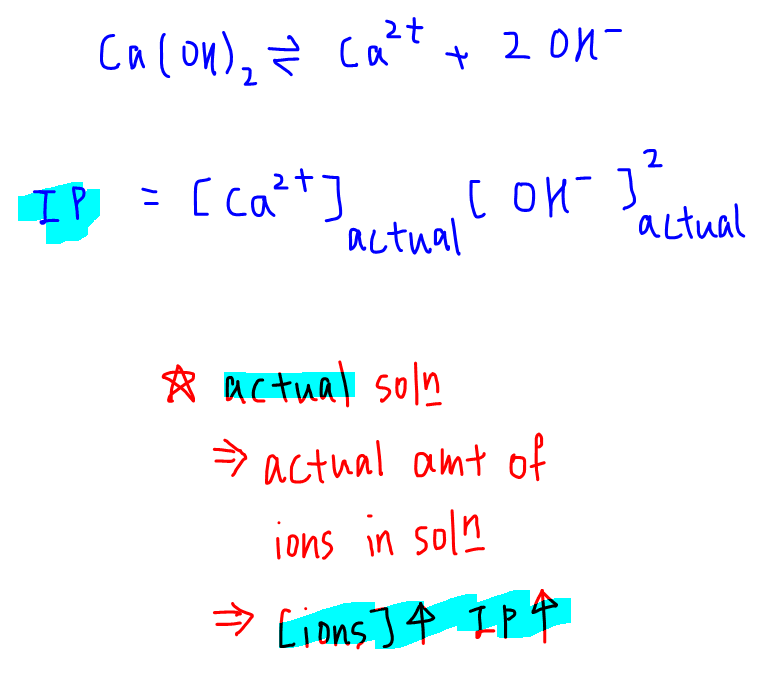
What Do Curly Brackets Mean
I learned that square brackets ex: $$ can be used to denote the concentration of water molecules in a fluid. The notation used much in equilibrium problems and such.
However, sometimes I have seen curly brackets being used in similar scenarios, but I never really understood the difference. When are curly brackets used and what do they mean?
I found this example from an exam where the problem is to see if a solution of $\ce$ in water is concentrated enough to result in precipitation.
Following is what how they solve it in the example:
Where $Q$ is the actual saturation of the solution. And the answer is that no precipitation occurs since $Q < K_\mathrm$ in this specific example.
So they seem to suggest that is something different than the concentration and that it can be approximated to the concentration.