What Is The Meaning Of Mw Abbreviation In Chemistry
What is MW definition ?
MW definition is “Molecular Weight”.
What does MW mean in Chemistry?
MW mean that “Molecular Weight” for Chemistry.
What is MW acronym ?
What is shorthand of Milliwatt ?
The shorthand of “Milliwatt” is MW.
What is the definition of MW acronym in Chemistry?
Definitions of MW shorthand is “Molecular Weight”.
What is the full form of MW abbreviation?
Full form of MW abbreviation is “Molecular Weight”.
What is the full meaning of MW in Chemistry?
Full meaning of MW is “Magnetized Water”.
What is the explanation for MW in Chemistry?
Explanation for MW is “Magnetized Water”.
What is the meaning of MW Abbreviation in Astrology ?
The site does not only include the meanings of the MW abbreviation in Chemistry. Yes, we know your main purpose is explanation of MW abbreviation in Chemistry. However, we thought that besides the meaning of the MW definitions in Chemistry, you can consider astrological information of MW acronym in Astrology. Therefore, the astrological explanation of each word in each MW abbreviation is also included.
MW Abbreviation in Astrology
Molecular Weights Of Polymers
Synthetic polymers consist of individual polymers with varying molecular weights. The properties of the polymers depend not only on the average molecular weight but also on the distribution of molecular weights as well. An example of a molecular weight distribution typical of free radical reactions is shown in Fig. 18.8. Many of the methods used to characterize polymers give average molecular weights that depend on the molecular weight distribution as well as the number average molecular weight. It becomes useful, as will be demonstrated shortly, to define several types of molecular weight distributions.
Figure 18.8. Typical distribution of molecular weights in polymers produced by free radical polymerization.
The first is a simple average where each molecule contributes equally to the average . Let Ni equal the number of the molecules with mass Mi . Using summation terminology for each fraction from i = 1 to , abbreviated here as , the number of molecules in each fraction multiplied by the molecular weight for each fraction are summed and the entire sum is divided by the total number of molecules, as shown in Eq. .
A second type of average gets around these difficulties it is the weight average, M
Shinichi Kitamura, Shiho Suzuki, in, 2020
What Is The Difference Between Molar Mass And Molecular Weight
It’s all about the units. Understanding the difference between molecular weight and molar mass all comes down to a difference of what is being counted and the units.
Molecular weight is the mass of a molecule of a substance. It can also be called molecular mass. The units for molecular weight are atomic mass units .
Molar mass is the mass of one mole of a substance. Molar mass is reported in grams per mole or g/mol.
Recommended Reading: Why Are There Different Branches Of Chemistry
What Does Mw Stand For Chemistry
We compiled queries of the MW abbreviation in Chemistry in search engines. The most frequently asked MW acronym questions for Chemistry were selected and included on the site.
We thought you asked a similar MW question to the search engine to find the meaning of the MW full form in Chemistry, and we are sure that the following Chemistry MW query list will catch your attention.
The Plot Thickens: Viscosity Average Molecularweight Mv
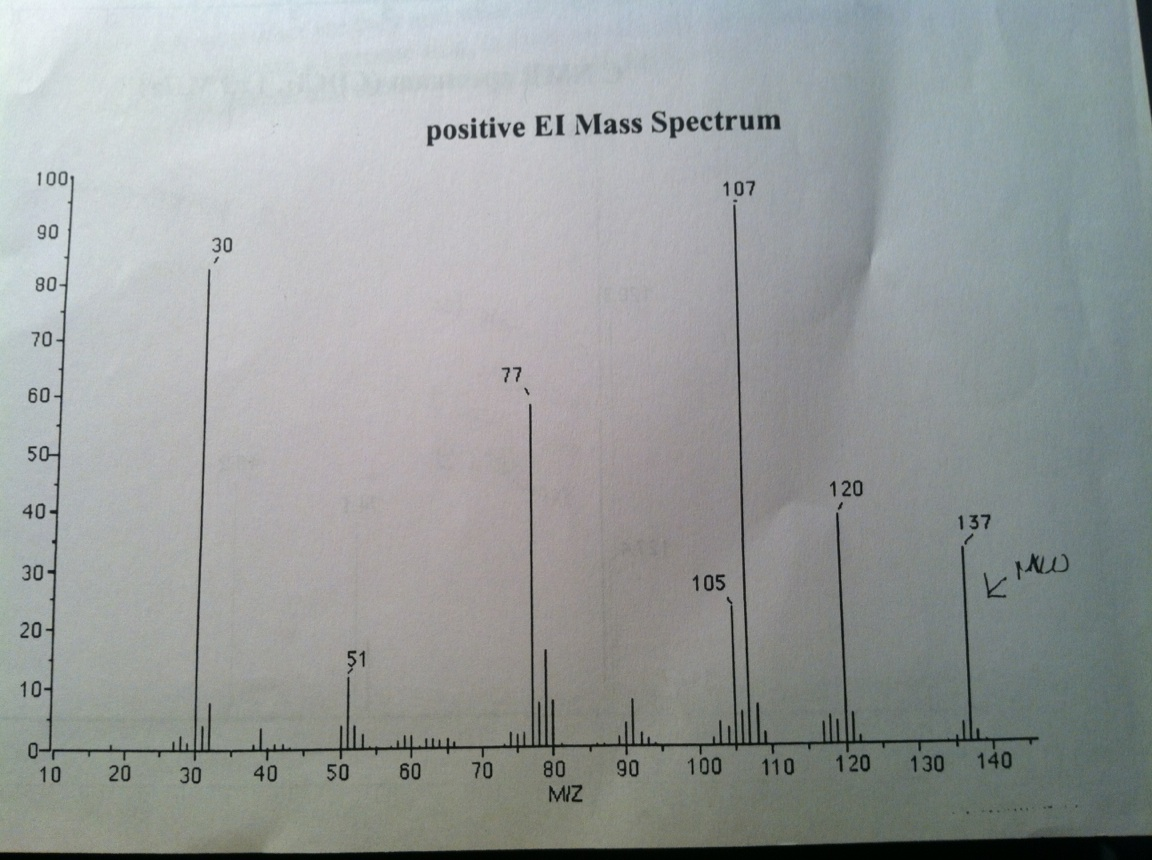
Molecular weight can also be calculated from the viscosity of a polymer solution. The principle is a simple one: Bigger polymers molecules make a solution more viscous than small ones do. Of course, the molecular weight obtained by measuring the viscosity is a different from either the number average or the weight average molecular weight. But it’s closer to the weight average than the number average. To read more about how wemeasure the viscosity average molecular weight, go read the dilute solution viscometry page.
Recommended Reading: How To Find Ksp Chemistry
How Do You Calculate The Molar Mass Of A Substance
Now that you already know how to calculate the molecular weight, calculating the molar mass is easy. Why? Once you know the molecular weight, you also know the molar mass.
The molecular weight of sodium is 22.99 amu. The molar mass of sodium is 22.99 grams per mol . It’s that simple!
So then how do you calculate the molar mass of water?
You need to know the molar mass of the elements that make up water. The molar mass of hydrogen is 1.008 g/mol as given by the period table. The molar mass of oxygen is 16.00 g/mol. So:
Remember, you have to account for the two parts of hydrogen per oxygen so that’s why there is a two in front of the molar mass of oxygen. Plugging in the molar mass of each element gives:
Which becomes:
The molar mass of water is 18.02 g/mol. You’ll note that this is the same number you got for the molecular weight, but the units are different.
Tips
-
Molecular weight is the mass of one molecule of a compound, while molar mass is the mass of one mole of a compound.
Related Articles
Abbreviations Starting With M
M3/H – Cubic Meters per HourmA – milliampereMADG – Moisture Activated Dry GranulationMAM – Methyl Azoxy MethanolMASER – Microwave Amplification by Stimulated Emission of RadiationMAX – MAXimumMDCM – Mechanistically Defined Chemical MixturesMDI – Methylene Diphenyl diIsocyanateme – mass of an electronME – Materials EngineeringMeV – Million electronVolt or MegaelectronVoltMF – Methyl FormateMIDAS – Molecular Interactions Dynamics And SimulationsMIG – Metal Inert GasMOAH – Mineral Oil Aromatic HydrocarbonMOH – Measurement of HardnessMSDS – Material Safety Data SheetMSG – MonoSodium Glutamate
Don’t Miss: Lesson 1.7 Practice A Geometry Answers
Measuring The Molecular Mass
The molecular mass can also be measured directly using mass spectrometry. In mass spectrometry, the molecular mass of a small molecule is usually reported as the monoisotopic mass, that is, the mass of the molecule containing only the most common isotope of each element. Note that this also differs subtly from the molecular mass in that the choice of isotopes is defined. The masses used to compute the monoisotopic molecular mass are found on a table of isotopic masses and are not the same as found on a typical periodic table. The average molecular mass is often used for larger molecules since molecules with many atoms are unlikely to be composed exclusively of the most abundant isotope of each element. A theoretical average molecular mass can be calculated using the standard atomic weights found on a typical periodic table, since there is likely to be a statistical distribution of atoms representing the isotopes throughout the molecule. This however may differ from the true average molecular mass of the sample due to natural variations in the isotopic distributions.
Converting Atoms To Moles
Reversing the calculation above, it is possible to convert a number of atoms to a molar quantity by dividing it by Avogadros number:
\frac}} \frac}}}}= y\text
This can be written without a fraction in the denominator by multiplying the number of atoms by the reciprocal of Avogadros number:
x \text\cdot\frac}\text} = y \text
For example, if scientists know there are 3.5 \cdot 10^ atoms in a sample, they can calculate the number of moles this quantity represents:
3.5\times 10^\text\cdot\frac} \text} = 5.81\text
Also Check: Sacred Geometry Moon Phases Tattoo Spine
How Do You Calculate Molecular Weight
First, you will need the mass of individual compounds. This number is found under the element on the period table. For example, the molecular weight of oxygen is 16.00 amu.
Once you know the mass of the individual components of a compound, you can find the molecular weight.
For example, the molecular weight of water is:
The mass of hydrogen is 1.008 amu, and the mass of oxygen is 16.00 amu. Plugging that in yields:
Adding the molecular weights of each element together gives:
Thus, the molecular weight of water is 18.02 amu.
Converting Between Mass Number Of Moles And Number Of Atoms
How many moles and how many atoms are contained in 10.0 g of nickel?
According to the periodic table, the atomic mass of nickel is 58.69 amu, which means that the molar mass of nickel is 58.69 g/mol. Therefore, we can divide 10.0 g of Ni by the molar mass of Ni to find the number of moles present.
Using dimensional analysis, it is possible to determine that:
10\text\times \frac}} = 0.170\text
To determine the number of atoms, convert the moles of Ni to atoms using Avogadros number:
0.170\text\times\frac \text}} = 1.02\times10^\text
Given a samples mass and number of moles in that sample, it is also possible to calculate the samples molecular mass by dividing the mass by the number of moles to calculate g/mol.
What is the molar mass of methane if there are 0.623 moles in a 10.0g sample?
\frac_4}_4} = 16.05 \text_4
The molar mass of CH4 is 16.05 g/mol.
Recommended Reading: Algebra 1 Age Word Problems
What Does Mw Mean In Chemistry
This page is about the meanings of the acronym/abbreviation/shorthand MW in the Academic & Science field in general and in the Chemistry terminology in particular.
Molecular Weight
Find a translation for Molecular Weight in other languages:
Select another language:
- Indonesia
What does MW mean?
- mw
- .mw is the Internet country code top-level domain for Malawi. In 2002 the IANA recommended that administration of the ccTLD be transferred to the Malawi Sustainable Development Network Programme from Computer Solutions LTDPrices for .mw domains are 100 USD for the first two years, followed by 50 USD per year.
From Empirical Formula To Molecular Formula
The empirical formula gives only the relative numbers of atoms in a substance in the smallest possible ratio. For a covalent substance, chemists are usually more interested in the molecular formula, which gives the actual number of atoms of each kind present per molecule. Without additional information, however, it is impossible to know whether the formula of penicillin G, for example, is C16H17N2NaO4S or an integral multiple, such as C32H34N4Na2O8S2, C48H51N6Na3O12S3, or n, where n is an integer. ).
Consider glucose, the sugar that circulates in our blood to provide fuel for the body and brain. Results from combustion analysis of glucose report that glucose contains 39.68% carbon and 6.58% hydrogen. Because combustion occurs in the presence of oxygen, it is impossible to directly determine the percentage of oxygen in a compound by using combustion analysis other more complex methods are necessary. Assuming that the remaining percentage is due to oxygen, then glucose would contain 53.79% oxygen. A 100.0 g sample of glucose would therefore contain 39.68 g of carbon, 6.58 g of hydrogen, and 53.79 g of oxygen. To calculate the number of moles of each element in the 100.0 g sample, divide the mass of each element by its molar mass:
Once again, the subscripts of the elements in the empirical formula are found by dividing the number of moles of each element by the number of moles of the element present in the smallest amount:
\ + \left + \left = 30.026 g \label\]
Also Check: Algebra 1 Eoc Fsa Practice Test
Synthetic Organic And Pharmaceutical Chemistry
Acetylation can be achieved using a variety of methods, the most common one being via the use of acetic anhydride or acetyl chloride, often in the presence of a tertiary or aromatic aminebase. A typical acetylation is the conversion of glycine to N-acetylglycine:
- H2NCH2CO2H + 2O CH3CNHCH2CO2H + CH3CO2H
Molecular Weight Versus Molecular Mass
Molecular weight is often used interchangeably with molecular mass in chemistry, although technically there is a difference between the two. Molecular mass is a measure of mass and molecular weight is a measure of force acting on the molecular mass. A more correct term for both molecular weight and molecular mass, as they are used in chemistry, would be “relative molecular mass”.
Read Also: Define Percent Error In Chemistry
Computing The Molecular Mass
The molecular mass can be calculated as the sum of the individual isotopic masses of all the atoms in any one molecule. This is possible because molecules are created by chemical reactions which, unlike nuclear reactions, have very small binding energiescompared to the rest mass of the atoms and therefore create a negligible mass defect. Note that the use of average atomic masses as found on a standard periodic table will result in an average molecular mass, whereas the use of isotopic masses will result in a molecular mass consistent with the strict interpretation of the definition, i.e. that of a single molecule. Note that any given molecule may contain any given combination of isotopes, so there may be multiple molecular masses for each chemical compound.
Mw Meaning In Chemistry
- And finally again and again search .
Also Check: Massachusetts Bay Colony Geography And Climate
Examples Of Chemistry In A Sentence
chemistrychemistrychemistrychemistry clevelandchemistry Washington Postchemistry Detroit Free Presschemistry CNNchemistrySan Diego Union-Tribunechemistry Chronchemistry BostonGlobe.comchemistryUSA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘chemistry.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Chemical Computations With Avogadros Number And The Mole
Avogadros number is fundamental to understanding both the makeup of molecules and their interactions and combinations. For example, since one atom of oxygen will combine with two atoms of hydrogen to create one molecule of water , one mole of oxygen will combine with two moles of hydrogen to make one mole of H2O.
Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units , so one mole of water weight 18.015 grams. This property simplifies many chemical computations.
If you have 1.25 grams of a molecule with molecular weight of 134.1 g/mol, how many moles of that molecule do you have?
1.25\text \times \frac}}=0.0093 \text
The Mole, Avogadro: This video introduces counting by mass, the mole, and how it relates to atomic mass units and Avogadros number.
Read Also: Who Is Paris Jackson’s Mother
What Is The Difference Betweet Molecular Mass And Molar Mass
See explanation.
Explanation:
Molecular mass is a term used to denote the mass of a single molecule and is measured in unified atomic mass units, #”u”# , much like atomic mass, which denotes the mass of a single atom.
Now, a unified atomic mass unit, which is defined as #1/12″th”# of the mass of a single, unbound carbon-12 atom, is equal to
#colorcolor”g”)color|)))#
Let’s take water, , as an example of how molecular mass works.
The molecular mass of water is equal to #”18.015 u”# , which means that a single water molecule has a mass of
#18.015 color)) * “g”)/))) = 2.99146 * 10^”g”#
So, if one molecule of water has a mass of #2.99146 * 10^”g”# , it follows that one mole of water, which contains #6.022 * 10^# molecules of water as given by Avogadro’s number, will have a mass of
#2.99146 * 10^”g”/))) * color)))/ ~~ “18.015 g mol”^#
This represents water’s molar mass, which denotes the mass of one mole of water molecules.
As you can see, you have
#colorcolor)color|)))#
Long story short, for a given compound, molecular mass tells you the mass of single molecule, while molar mass tells you the mass of a mole of said compound.
#color#
As given to you, the molecular mass, #M_r# , is most likely the relative molecular mass, sometimes called molecular weight, which is defined as molecular mass divided by #1/12″th”# of the mass of a single, unbound carbon-12 atom.
#”relative molecular mass” = “molecular mass”/”1 u”#
For water, the relative molecular mass is
#M_r = )))/))) = 18.015#
Example: Average Molecular Mass Versus Molecular Mass Versus Molar Mass
The molar mass of a substance is the mass of 1 mol of the substance. This has a numerical value which is the average molecular mass of the molecules in the substance multiplied by Avogadro’s constant approximately 6.022*1023. Its SI unit is kg/mol, although more usually the unit g/mol is used because in those units the numerical value equals the average molar mass in units of u.
Conversion Factor of average molecular mass to molar mass:
- molar mass = average molecular mass * *
- or
- molar mass in g/mol= average molecular mass in u
The average atomic mass of natural hydrogen is 1.00794 u and that of natural oxygen is 15.9994 u therefore, the molecular mass of natural water with formula H2O is + 15.9994 u = 18.01528 u. Therefore, one mole of water has a mass of 18.01528 grams. However, the exact mass of hydrogen-1 is 1.00783, and the exact mass of oxygen-16 is 15.9949, so the mass of the most common molecule of water is 18.01056 u. The difference of 0.00472 u or 0.03% comes from the fact that natural water contain traces of water molecules containing, oxygen-17, oxygen-18 or hydrogen-2 atoms. Although this difference is trivial in bulk chemistry calculations, it can result in complete failure in situations where the behavior of individual molecules matters, such as in mass spectrometry and particle physics .
Don’t Miss: What Is Figure Ground In Psychology