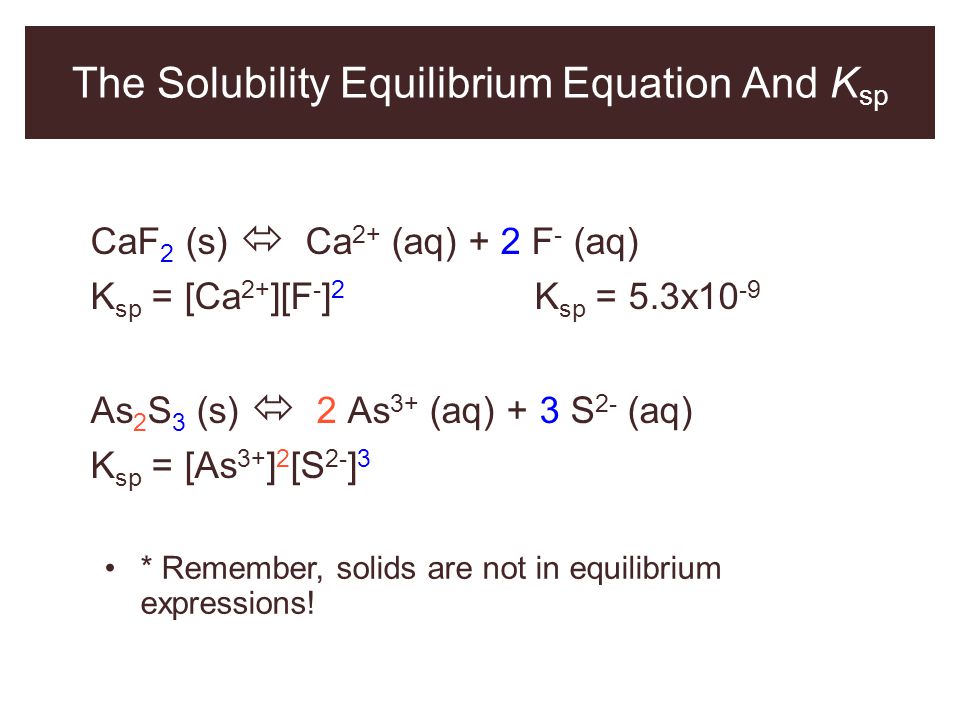
Relating Solubilities To Solubility Constants
The of a solid is expressed as the concentration of the “dissolved solid” in a saturated solution. In the case of a simple 1:1 solid such as AgCl, this would just be the concentration of Ag+ or Cl in the saturated solution. But for a more complicated stoichiometry such as as silver chromate, the solubility would be only one-half of the Ag+ concentration.
For example, let us denote the solubility of Ag2CrO4 as S mol L1. Then for a saturated solution, we have
- \
Substituting this into Eq 5b above,
thus the solubility is \.
Note that the relation between the solubility and the solubility product constant depends on the stoichiometry of the dissolution reaction. For this reason it is meaningless to compare the solubilities of two salts having the formulas A2B and AB2, say, on the basis of their Ks values.
Note
It is meaningless to compare the solubilities of two salts having different formulas on the basis of their Ks values.
Example \
The solubility of CaF2 at 18°C is reported to be 1.6 mg per 100 mL of water. Calculate the value of Ksunder these conditions.
Solution
moles of solute in 100 mL; S = 0.0016 g / 78.1 g/mol = \ mol
\^2 = ^2 = 4 × ^3 = 3.44 \times 10^\]
Example \
Estimate the solubility of La3 and calculate the concentration of iodate in equilibrium with solid lanthanum iodate, for which Ks = 6.2 × 1012.
Solution
The equation for the dissolution is
If the solubility is S, then the equilibrium concentrations of the ions will be
= 3S = 2.08 × 105
Solution
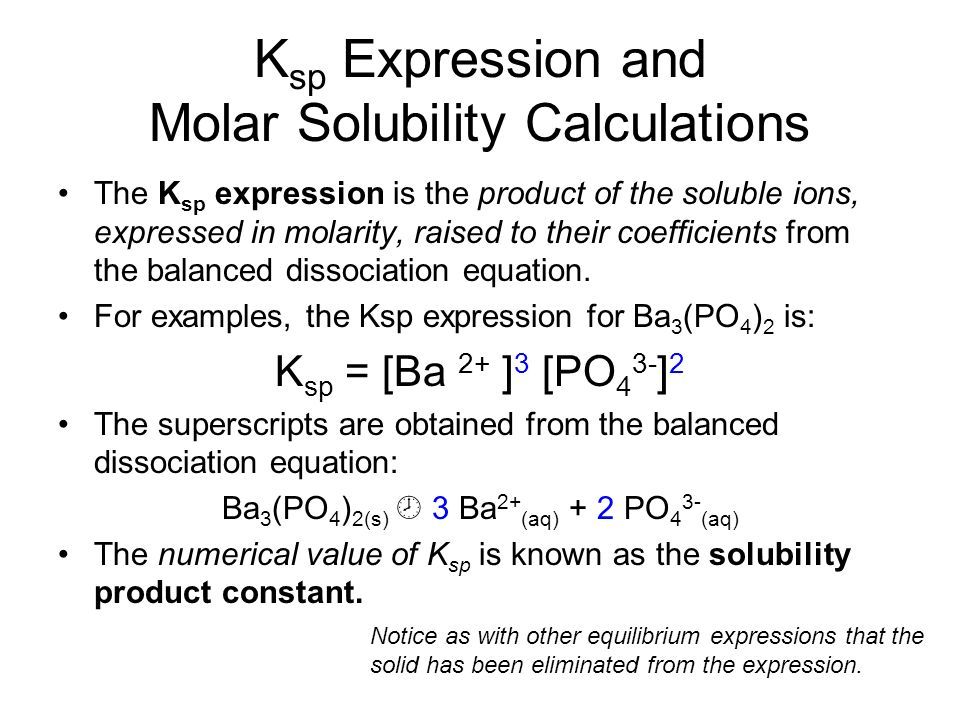
Conversion Of Ksp To Solubility
| Compound | |
| Ca 3 2 | 1.2 × 10 -26 |
| ZnS | 3.0;× 10 -23 |
The known;Ksp;values from the Table above can be used to calculate the solubility of a given compound by following the steps listed below.
The;Ksp;of calcium carbonate is 4.5;×;10 -9 . We begin by setting up an ICE table showing the dissociation of CaCO 3 into calcium ions and carbonate ions. The variable; will be used to represent the molar solubility of CaCO 3 . In this case, each formula unit of CaCO 3 yields one Ca 2+ ion and one CO 3 2 ion. Therefore, the equilibrium concentrations of each ion are equal to .
The;Ksp;expression can be written in terms of; and then used to solve for .
The concentration of each of the ions at equilibrium is 6.7;×;10 -5 ;M. We can use the molar mass to convert from molar solubility to solubility.
When the;Ksp;expression is written in terms of , we get the following result for the molar solubility.
The Table below shows the relationship between;Ksp;and molar solubility based on the formula.
| Compound Type |
What Is The Difference Between Solubility And Solubility Product Constant
The solubility of a substance in a solvent is the total amount of the solute that can be dissolved in the solvent at equilibrium. On the other hand, the solubility product constant is an equilibrium constant that provides insight into the equilibrium between the solid solute and its constituent ions that are dissociated across the solution.
You May Like: When Was Geometry Dash Made
Finding Ksp From Molar Solubility
Given molar solubility for the ions in question and the balanced equation, you can find the Ksp.
Consider the following equation:
The solubility product is written as such:
Remember: The must be raised to the second power due to the coefficient in the balanced equation.
Now, given that the molar solubility is 2.2 x 10-3 M, you can plug this into the equation for both and . Remember that the molar solubility must be the same for both since they come from the same compound.
Solubility is affected by multiple factors.
Related Articles
Ksp Chemistry: Complete Guide To The Solubility Constant
Are you learning chemistry but dont quite understand the solubility product constant or want to learn more about it? Not sure how to calculate molar solubility from $K_s_p$? The solubility constant, or $K_s_p$, is an important part of chemistry, particularly when youre working with solubility equations or analyzing the solubility of different solutes. When you have a solid grasp of $K_s_p$, those questions become much easier to answer!
In this $K_s_p$ chemistry guide, well explain the $K_s_p$ chemistry definition, how to solve for it , which factors affect it, and why its important. At the bottom of this guide, we also have a table with the $K_s_p$ values for a long list of substances to make it easy for you to find solubility constant values.
Read Also: What Not To Do In The Chemistry Lab
How Do You Purify Water
Purification of water for drinking and other uses is a complicated process. Heavy metals need to be removed, a process accomplished by addition of carbonates and sulfates. Lead contamination can present major health problems, especially for younger children. Lead sulfates and carbonates are very insoluble, so will precipitate out of solution very easily.
Significance Of Solubility Product
Solubility depends on a number of parameters amongst which lattice enthalpy of salt and solvation enthalpy of ions in the solution are of most importance.
- When a salt is dissolved in a solvent the strong forces of attraction of solute must be overcome by the interactions between ions and the solvent.
- The solvation enthalpy of ions is always negative which means that energy is released during this process.
- The nature of the solvent determines the amount of energy released during solvation that is solvation enthalpy.
- Non-polar solvents have a small value of solvation enthalpy, meaning that this energy is not sufficient to overcome the lattice enthalpy.
- So the salts are not dissolved in non-polar solvents. Hence, for salt to be dissolved in a solvent, its solvation enthalpy should be greater than its lattice enthalpy.
- Solubility depends on temperature and it is different for every salt.
Salts are classified on the basis of their solubility in the following table.
| Category I |
| Solubility < 0.1M |
Read Also: What Does Abiotic Mean In Biology
To Predict If A Precipitate Will Form In Reactions
When we know the $K_s_p$ value of a solute, we can figure out if a precipitate will occur if a solution of its ions is mixed. Below are the two rules that determine the formation of a precipitate.
- Ionic product > $K_s_p$ then precipitation will occur
- Ionic product < $K_s_p$ then precipitation will not occur
How Do You Solve The Ksp Equations
They all follow a basic pattern, which I will describe and demonstrate below…
Explanation:
To illustrate the method, I will use a generic insoluble solid #XY_2#
When dissolved in water, our compound dissolves according to
#XY_2 rarr X^ + 2 Y^-# ##
# ^2 = 4xx10^#
Here’s the solution:
First, let the variable #x# represent the solubility of the solid . What we must do is express both ion concentrations in terms of #x#
First, since each formula unit of #XY_2# ion, the equilibrium concentration of #X^#
ions, menaing the equilibrium concentration of #Y^-#
Placing these variables into the #K_#
To solve this, note that #^2#
Divide each side by 4, then take the cube root:
#x=root #
This means the solubility is #1xx10^# and the ion concentrations are # = 1xx10^ M and = 2xx10^# at equilibrium.
Ksp is a particular form of Equilibrium in solution. Use an I.C.E. chart to correctly determine the quantities of reactants and products before using th equations.
Explanation:
Some simpler solutions may be calculated directly. For example, to find the concentration of aluminum ion inn equilibrium with hydroxide, given the Ksp. # # #Al_3 ” solution with” = 2.9 * 10^# M
#Al_3 Al^ + 3OH^-#
#Ksp = ^3 = 1.8 * 10^#
Using the given value of the hydroxide ion concentration, the equilibrium concentration of aluminum ion is:
# = /^3 = 1.8 * 10^#
# = )/)^3 #
# = )/)^3 #
#= 0.738 * 10^#
# = 7.38 * 10^ M #
Read Also: How To Login To Imagine Math
Conversion Of Solubility To Ksp
Solubility is normally expressed in g/L of saturated solution. However, solubility can also be expressed as the moles per liter. Molar solubility is the number of moles of solute in one liter of saturated solution. In other words, the molar solubility of a given compound represents the highest molarity solution that is possible for that compound. The molar mass of a compound is the conversion factor between solubility and molar solubility. Given that the solubility of Zn 2 is 4.2;×;10 -4 ;g/L, the molar solubility can be calculated as shown below:
Solubility data can be used to calculate the;Ksp;for a given compound. The following steps need to be taken.
Sample Problem: Calculating;Ksp;from Solubility
The solubility of lead fluoride is found experimentally to be 0.533 g/L. Calculate the;Ksp;for lead fluoride.
Step 1: List the known quantities and plan the problem .
Known
- solubility of PbF 2 = 0.533 g/L
- molar mass = 245.20 g/mol
Determine Ksp From Solubility
Problem:
Lead sulfate is a key component in lead-acid car batteries. Its solubility in water at 25°C is 4.25×103;g/100. mL solution. What is the;Ksp;of PbSO4?
Plan:
Given solubilities, Find Ksp.1. > write equation and expression2. > convert solubility to molar solubility3. > determine molarities of the ions4. > Substitute into Ksp
Solve!
1. Write equation for PbSO4;
2. Molar Solubility = moles per litre.
3. Determine molarities of the ions... Since 1 mol of each ion form when the solid is dissolved:
4. Substitute into Ksp expression
Answer
Also Check: Math Caching Algebra 1 Answers
How Is Baking Soda Made
Baking soda is prepared by bubbling carbon dioxide gas through a solution of ammonia and sodium chloride. Ammonium carbonate is first formed which then reacts with the NaCl to form sodium bicarbonate and ammonium chloride. The sodium bicarbonate is less soluble than the other materials, so it will precipitate out of solution.
To Find The Solubility Of Solutes
Wondering how to calculate molar solubility from $K_s_p$? Knowing the value of $K_s_p$ allows you to find the solubility of different solutes. Heres an example: The $K_s_p$ value of $Ag_2SO_4$ ,silver sulfate, is 1.4×$10^^5$. Determine the molar solubility.
First, we need to write out the dissociation equation: $K_s_p$=$ ^2$ $$
Next, we plug in the $K_s_p$ value to create an algebraic expression.
1.4×$10^^5$= $^2$ $$
1.4×$10^^5$= $4x^3$
$x$==1.5x$10^^2$ M
$2x$= =3.0x$10^^2$ M
Recommended Reading: Algebra Road Trip Project Answer Key
The Common Ion Effect
It has long been known that the solubility of a sparingly soluble ionic substance is markedly decreased in a solution of another ionic compound when the two substances have an ion in common. This is just what would be expected on the basis of the Le Châtelier Principle; whenever the process
is in equilibrium, addition of more fluoride ion will shift the composition to the left, reducing the concentration of Ca2+, and thus effectively reducing the solubility of the solid. We can express this quantitatively by noting that the solubility product expression
\^2 = 1.7 \times 10^ \label\]
must always hold, even if some of the ionic species involved come from sources other than CaF2. For example, if some quantity x of fluoride ion is added to a solution initially in equilibrium with solid CaF2, we have
- \
so that
\^2 = S ^2 . \label\]
xSS
The plots shown below illustrate the common ion effect for silver chromate as the chromate ion concentration is increased by addition of a soluble chromate such as Na2CrO4.
What’s different about the plot on the right? If you look carefully at the scales, you will see that this one is plotted logarithmically Notice how a much wider a range of values can display on a logarithmic plot. The point of showing this pair of plots is to illustrate the great utility of log-concentration plots in equilibrium calculations in which simple approximations can yield straight-lines within the range of values for which the approximation is valid.
Example \
To Understand The Common Ion Effect
$K_s_p$ also is an important part of the common ion effect. The common ion effect states that when two solutions that share a common ion are mixed, the solute with the smaller $K_s_p$ value will precipitate first.
For example, say BiOCl and CuCl are added to a solution. Both contain $Cl^$ ions. BiOCls $K_s_p$ value is 1.8×$10^^31$ and CuCls $K_s_p$ value is 1.2×$10^^6$. BiOCl has the smaller $K_s_p$ value, so it will precipitate before CuCl.
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
Solving For $k_s_p$ With Solubility
In order to calculate a value for $K_s_p$, you need to have molar solubility values or be able to find them.
Question: Determine the $K_s_p$ of AgBr , given that its molar solubility is 5.71 x $10^^7$ moles per liter.
First, we need to write out the two equations.
AgBr $Ag^$ + $Br^$
$K_s_p$ =
Now, since in this problem we’re solving for an actual value of $K_s_p$, we plug in the solubility values we were given:
$K_s_p$ = = 3.26 x $10^^13$
The value of $K_s_p$ is 3.26 x $10^^13$
What Is Solubility Product Ksp
The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. It is denoted by the symbol Ksp.
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
Solubility is defined as a property of a substance called solute to get dissolved in a solvent in order to form a solution.The solubility of ionic compounds in water varies to a great deal. Some compounds are highly soluble and may even absorb moisture from the atmosphere whereas others are highly insoluble.
You May Like: What Is The Molecular Geometry Of Ccl4
Solubility Product Constant Table
Below is a chart showing the $K_s_p$ values for many common substances. The $K_s_p$ values are for when the substances are around 25 degrees Celsius, which is standard. Because the $K_s_p$ values are so small, there may be minor differences in their values depending on which source you use. The data in this chart comes from the University of Rhode Islands Department of Chemistry.
| Substance |
How Do You Calculate $k_s_p$
In this section, we explain how to write out $K_s_p$ chemistry expressions and how to solve for the value of $K_s_p$. For most chemistry classes, youll rarely need to solve for the value of $K_s_p$; most of the time youll be writing out the expressions or using $K_s_p$ values to solve for solubility .
Also Check: How Do Noise Cancelling Headphones Work Physics
Finding Molar Solubility From Ksp
Given that the Ksp for AgCl is 1.7 x 10-10, you can find the molar solubility, or the concentration of either ion in the solution:
Now what? There are two variables: and , so how can you solve for the molar solubility? Since these ions come from the same solid, it is impossible that there is more of one than the other. This means that = . So:
Taking the square root of both sides allows you to solve for the molar solubility:
Thus, the molar solubility of both Ag+ and Cl- is thus 1.3 x 10-5 M.
How Do You Find Molar Concentration From Ksp
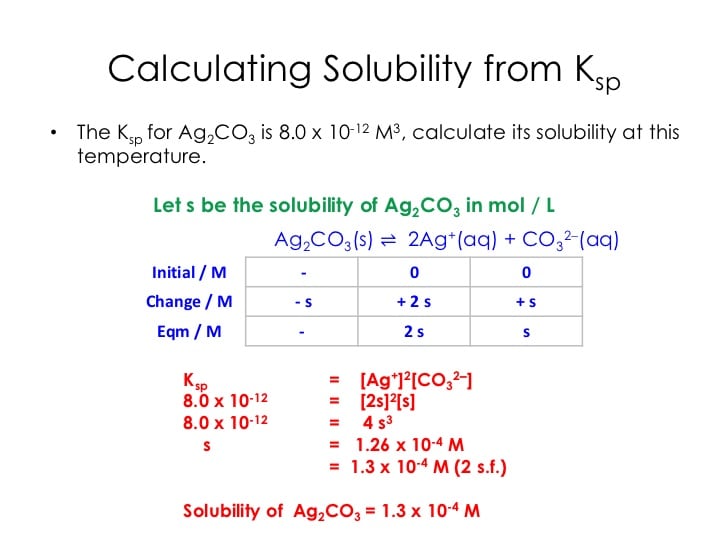
molar solubilitymol
Next we write out the expression for Ksp , then “plug in” the concentrations to obtain the value for Ksp. Let’s do an example: The solubility of Ag2CrO4 in water is 1.31 x 10-4 moles/L. Calculate the value of Ksp . Using mole ratios, the will go up by = 2.62 x 10-4 moles/L.
Subsequently, question is, what affects KSP? Solubility is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature. There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
Subsequently, question is, what is the unit of KSP?
Units of Solubility ProductSolubility products have units of concentration raised to the power of the stoichiometric coefficients of the ions in the equilibrium. So the solubility product of PbCl2 has units of M3 or mol3 dm-9.
What is the formula for calculating solubility?
Solubility indicates the maximum amount of a substance that can be dissolved in a solvent at a given temperature. Such a solution is called saturated. Divide the mass of the compound by the mass of the solvent and then multiply by 100 g to calculate the solubility in g/100g .
Read Also: Which Founding Contributors To Psychology Helped Develop Behaviorism
Relationship Between Solubility And Ksp
Learning Objectives
- Quantitatively related \ to solubility
Considering the relation between solubility and \ is important when describing the solubility of slightly ionic compounds. However, this article discusses ionic compounds that are difficult to dissolve; they are considered “slightly soluble” or “almost insoluble.” Solubility product constants ) are given to those solutes, and these constants can be used to find the molar solubility of the compounds that make the solute. This relationship also facilitates finding the \ of a slightly soluble solute from its solubility.