Atherosclerosis And Coronary Artery Disease
Surfactant system is probably the most genetically consistent system amongst all vertebrate species. The differences in interfacial tension of plasma and serum amongst various species including human, canine, porcine and equine are relatively small., Albumin with a concentration of 35-50 g/L is the main active compound of serum. Surface tension is sensitive to pH values as well as presence of inorganic and organic electrolytes, carbohydrates and other surfactants. Dynamic surface tension measurements of serum can be used as an indicator of some pathologic disturbances.
There are sex related differences in the surface tension of human serum . High values of equilibrium surface tension in female are due to the low physiological content of albumin, fatty acids and phospholipids, carbohydrate compound, lipoproteins, fibronectin and enzymes., Due to the metabolism of proteins and lipids and alterations in the surfactant concentration of biological fluids, the surface tension of serum increases with the age.,
What Is Surface Tension
Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. This attraction of molecules towards one another is known as an intermolecular force. In any liquid substance, the molecules are in constant random motion, and are constantly being rearranged. In the midst of the liquid, all of the molecules are being pulled by other molecules in every direction. However, at the surface, where there is only air above the liquid, the molecules are only being pulled laterally and downwards, by the molecules beside and below them, respectively.
This downward pull on the surface-level molecules causes them to be pulled in tighter to one another, compressing into a more stable, aligned arrangement. This tighter row of surface molecules forms something of an elastic membrane on the surface of the liquid. The molecules are more tightly arranged and are smoothly lined up beside one another, unlike the more chaotic molecular arrangements below.
Surface Tension And Cohesiveness
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter , is high and shows a slight increase as the temperature falls from 100 to 0 °C . The molecules of water are strongly attracted to each other through their cohesiveness . The properties of surface tension and cohesiveness work together in water in shaping the small rounded water droplets seen on a table top or a car windshield. The same properties help to form the slightly flattened to spherically-shaped raindrops as they fall through the air.
The primary force for restoring larger wind-generated surface and internal waves of lakes is gravity, but the primary force for restoring the much smaller capillary waves or ripples on a lake’s surface seems to be surface tension of the water itself.
One of the easiest ways of getting popcorn into your mouth is by touching your tongue to some popcorn in a container. Here again it is the surface tension of the water on your tongue that lets you hold on to the light popcorn easily.
You May Like: How Many Subfields Of Psychology Are There
Pulmonary Surfactant And Breathing Cycle
Airway is the main port of entry for pathogens and allergens. For this reason both upper airways and lower airways present an epithelium lining cell that secret mucus and pulmonary surfactant, respectively. Respiratory mucus represents a complex mixture of water, protein and glycoproteins, lipids and salts, which provides viscoelastic properties as a physical barrier for the entry of pathogens and allergens during breathing cycles. Pulmonary surfactant has been delineated as a surface-active material that coats the alveolar space of human lung to reduce the surface tension and prevent collapse of the alveoli during breathing it also plays a significant part in the lungs defense against bacterial or chemical invasion. PS is composed of lipids ) and surfactant associated proteins . The most abundant lipid with surface tension lowering properties is dipalmitoylphosphatidylcholine , ~60-70% wt%. DPPC is necessary to achieve surface tension of near zero mN/m during the film compression and expiration. Surfactant specific proteins include SP-A, SP-B, SP-C and SP-D. SP-A and SP-D are hydrophilic proteins with no surface properties. SP-B and SP-C have low molecular weight, hydrophobic molecules which lower surface tension and prevent the alveolar collapse at the end of expiration. Air pollutants can significantly interfere with the ability of lung surfactants to decrease the surface tension during breathing cycles.,
Surface Tension: Definition Explanation And Methods
In this article we will discuss about Surface Tension:- 1. Definition of Surface Tension 2. Explanation for Surface Tension 3. Method of Determination 4. Factors Affecting 5. Gibbs-Thomson Principle 6. Physiological Importance.
Contents:
1. Definition of Surface Tension
:
The force with which the surface molecules are held together is called surface tension.
2. Explanation for Surface Tension:
The interior molecules of a homogeneous liquid are equally attracted in all directions by surrounding molecules.
They are free to move in all directions. But the molecules in the surface of the liquid are attracted downward and sideways but not upward .
As a result, the molecules of the surface are not so free to move. They are held together and form a membrane over the surface of the liquid.
Therefore, when finely powdered sulphur or other non-wetting powders are sprinkled upon water, they do not sink but are suspended on the surface.
A great part of the energy required to convert a liquid into a gas is essential to overcome surface tension and drag the molecules free from the surface of the liquid.
There is also an interfacial tension which is biochemically very important, especially in the process of adsorption.
This tension lies at the boundary between immiscible liquids, e.g., oil drops emulsified in water.
The tension is due to unequal attraction of the film molecules as compared with the molecules in the interior of the liquid.
3. Method of Determination of Surface Tension:
Recommended Reading: Did Michael Jackson Have Biological Kids
Infection Of The Human Cervix
Human cervical mucus contains mucin and soluble proteins. Mucin is secreted from the epithelium cells of the cervix and plays an important role in the physicochemical properties of cervical mucus including viscosity and surface tension. Mucin acts as a lubricant to keep pathogens out. A relationship has been found between the surface tension of the mucous and the permeation rate of microbicides. Cervical compounds can be used in the treatment of dry vagina or as microbicidal agents against Sexually Transmitted Disease pathogens. The degree of irritation of these compounds depends upon their surface tension. Compounds with low surface tension have lower binding of the membrane to the mucosa proteins and thus will cause less cervical irritation.
Pressure Inside A Soap Bubble
To consider the pressure inside the soap bubble, we consider the radius R of the bubble and also the surface tension, gamma, of the liquid .
We begin by assuming no external pressure . You then consider a cross-section through the center of the bubble.
Along this cross section, ignoring the very slight difference in inner and outer radius, we know the circumference will be 2piR. Each inner and outer surface will have a pressure of gamma along the entire length, so the total. The total force from the surface tension is, therefore, 2gamma .
Inside the bubble, however, we have a pressure p which is acting over the entire cross-section pi R2, resulting in a total force of p.
Since the bubble is stable, the sum of these forces must be zero so we get:
2 gamma =porp= 4 gamma / R
Obviously, this was a simplified analysis where the pressure outside the bubble was 0, but this is easily expanded to obtain the difference between the interior pressure p and the exterior pressure pe:
p – pe= 4 gamma / R
Also Check: What Does Endpoint Mean In Geometry
Surface Tension Facts For Kids
| Navier · Newton · Stokes |
Surface tension is an effect where the surface of a liquid is strong. The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in a ball shape. Some small things can float on a surface because of surface tension, even though they normally could not float. Some insects can run on the surface of water because of this. This property is caused by the molecules in the liquid being attracted to each other , and is responsible for many of the behaviors of liquids.
Surface tension has the dimension of force per unit length, or of energy per unit area. The two are equivalentbut when referring to energy per unit of area, people use the term surface energywhich is a more general term in the sense that it applies also to solids and not just liquids.
In materials science, surface tension is used for either surface stress or surface free energy.
Cohesive And Adhesive Forces
There are several other important concepts that are related to surface tension. The first of these is the idea of cohesive and adhesive Forces. Cohesive forces are those that hold the body of a liquid together with minimum surface area and adhesive forces are those that try to make a body of a liquid spread out. So if the cohesive forces are stronger than the adhesive forces, the body of water will maintain its shape, but if the opposite is true than the liquid will be spread out, maximizing its surface area. Any substance that you can add to a liquid that allows a liquid to increase its surface area is called a wetting agent.
Also Check: Why Are There Different Branches Of Chemistry
Why Is Surface Tension Important
While this property of liquids is certainly interesting, it doesnt seem to play a very big role in our daily lives, but that is where you are wrong. Apart from watching cool videos of perfectly round water droplets falling in slow motion or water striders kicking themselves along at 2 meters/second by skimming along the surface of a lake, why does surface tension matter?
Water striders.
In certain industries, surface tension is an easier metric for the contamination of products. Since surface tension is determined at a molecular level, any change to the component liquids, surfactants, fuels or compounds in a liquid would result in a change in the surface tension. If the surface tension of a perfectly pure composition is known, any variation from that would reveal some level of contamination. This may seem like an abstract application of surface tension, but it shows how even the simplest things can have the biggest impact in science. Interestingly enough, the effect of impurities on surface tension was first discovered by Agnes Pockelsa woman fascinated by physics but denied access to educationwhile doing dishes in her kitchen!
What Is Surface Tension With Example
surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This phenomenon can be observed in the nearly spherical shape of small drops of liquids and of soap bubbles. The razor blade is not floating: if pushed through the surface, it sinks through the water.
Recommended Reading: Fsa Warm Ups Grade 5
Faqs About Surface Tension
Q.1. Is there any difference in surface tension values among tap water, distilled water?
Answer: The surface tension value depends on the number of impurities there are in the water. So if we observe clearly there are more impurities in the tap water than the distilled water.
Q.2. Why rain waters form beads on the surface of the leaf?
Answer: As the surface of the leaf is waxy so the water adheres weakly to wax and strongly to itself hence they forms clusters into drops. The surface tension water gives them spherical shapes.
Q.3. How water striders walk on the water?
Answer: Objects that are less denser than that of water can float on the surface of the water. The water striders use the surface tension of the water to walk on the surface of the water.
Q.4. What are the units of surface tension?
The units of surface tension are Newton per metre and Dyn/cm .
Q.5. What are the methods of measurement of surface tension?
Answer: We can measure the surface tension by using these methods-
Q.6. Why raindrops are spherical?
Answer: The raindrops are spherical because of the cohesive forces between the rainwater molecules and the surface tension of the water molecules.
Q.7. Which are the main forces act to create surface tension?
Answer: The main forces are the cohesive force and adhesive force.
Q.8. What is the surface tension of water at boiling point?
Answer: The surface tension of water is zero at boiling temperature.
Surface Tension Holes Experiment

Youll need
A big bowl of water
Some ground pepper or any other ground product with colour
Washing up liquid
Method
Once the water settles, sprinkle the ground pepper over the top.
In the middle of the bowl drip some washing up liquid and watch what happens.
You should see a hole appear in the centre as the pepper moves outwards. This is your surface tension hole!
Don’t Miss: Geometry Unit 1 Test Answer Key
Water Is An Excellent Solvent
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solventa substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt mixed in water , the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
Figure 3. When table salt is mixed in water, spheres of hydration form around the ions.
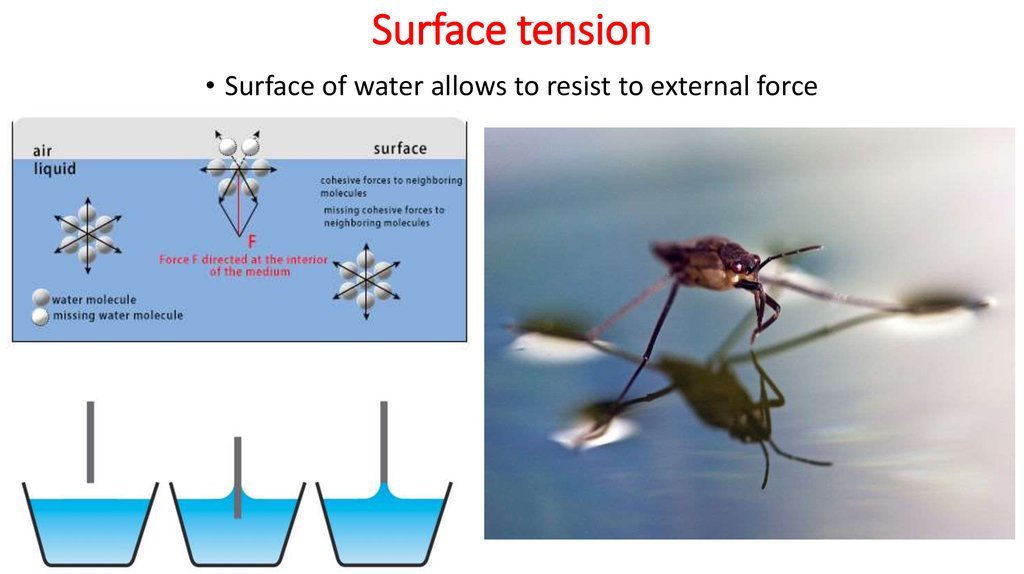
Cohesion And Surface Tension
The cohesive forces between molecules in a liquid are shared with all neighboring molecules. Those on the surface have no neighboring molecules above and, thus, exhibit stronger attractive forces upon their nearest neighbors on and below the surface. Surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water molecules.
You May Like: What Is Abiotic In Biology
Renal And Genitourinary Diseases
Urine constituents comprise of over 150 chemicals including electrolytes, nitrogenous compounds, vitamins, hormones, organic acids, and miscellaneous organic compounds. None of these normal constituents have any considerable effect on the urine surface tension. Urea, uric acid, creatinine, and acetone do not alter the surface tension. The urinary constituent that influences the surface tension includes bile salt and sodium chloride. Urine exhibits inverse relationship between the surface tension and the bile salt concentration. In other words surface tension of urine decreases with age and certain conditions such as acidity and jaundice. The surface tension characteristics of urine depend on the persons sex, age and diet . These differences may be due to various levels of eicosanoids, palmitic and hyaluronic acids, urea, creatinine and uric acid contents. It is found that increased levels of calcium in the urine can potentially increase bacterial adhesion to uroepithelial cells.,-
Surface Tension And Water
Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the environment and people. Find out all about surface tension and water here.
Water Science School HOME Water Properties topics
Surface Tension: “The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules.”
It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. The high surface tension helps the paper clip – with much higher density – float on the water.
The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface of a glass of water do not have other water molecules on all sides of them and consequently they cohere more strongly to those directly associated with them . It is not really true that a “skin” forms on the water surface the stronger cohesion between the water molecules as opposed to the attraction of the water molecules to the air makes it more difficult to move an object through the surface than to move it when it is completely submersed. .
Read Also: Algebra Age Problems
Examples Of Surface Tension
Water striders are able to walk on top of water due to a combination of several factors. Water striders use the high surface tension of water and long, hydrophobic legs to help them stay above water.
Water striders use this surface tension to their advantage through their highly adapted legs and distributed weight. The legs of a water strider are long and slender, allowing the weight of the water strider body to be distributed over a large surface area. The legs are strong, but have flexibility that allows the water striders to keep their weight evenly distributed and flow with the water movement. Hydrofuge hairs line the body surface of the water strider.