Saturated And Unsaturated Molecules
In the lab, saturation may be thought of as the point when a solution cannot dissolve anymore of a substance added to it. In terms of degrees of unsaturation, a molecule only containing single bonds;with no rings is considered saturated.
| ; CH3CH2CH3 |
Unlike saturated molecules, unsaturated molecules contain double bond, triple bond and/or ring.
| ;; CH3CH=CHCH3 |
Related Questions And Answers
What does degree of unsaturation of 4 mean?
What are the two kinds of bonds found in benzene?
How is DBE calculated?
How many pi bonds are in a triple bond?
What is the degree of unsaturation of Cyclobutene?
How do you identify sigma and pi bonds?
What is the degree of unsaturation present in DDT?
What is the use of degree of unsaturation?
How do you calculate Sodar?
What does a degree of unsaturation of 0 mean?
What is a sigma and pi bond?
How does nitrogen affect degrees of unsaturation?
How many single and double bonds are in benzene?
What kind of bonds does benzene have?
What is the bond angle of benzene?
What does a sigma bond look like?
How many sigma bonds are in benzene?
Nitrogen Is A Little Bit Tricky
Weve saved nitrogen for last, because its a bit weird.;;Try to;see the pattern of how nitrogen affects our formula.
OK, you might have noticed that we cant ignore nitrogen like we did oxygen.
The way to make the equation work with nitrogen is to add the nitrogen count to the right hand side of the equation, like this:
Try it with cadaverine which contains no double bonds or rings.
Read Also: Example Of Span Linear Algebra
Presentation On Theme: Spectroscopy Sl Chemistry Topic Ihd Index Of Hydrogen Deficiency Used To Determine From A Molecular Formula The Number Presentation Transcript:
1 SPECTROSCOPY SL Chemistry Topic 11.3
2 IHD Index of hydrogen deficiency Used to determine from a molecular formula the number of rings or multiple bonds in a molecule. IHD is used to calculate number of rings or bonds in a structure, where: A double bond is one degree of unsaturation A triple bond is two degrees of unsaturation A ring is counted as one degree of unsaturation An aromatic ring is counted as four degrees of unsaturation
3 What is the IHD for ecstasy, shown below?
4 IHD from molecular formula IHD from a generic molecular formula C c H h N n O o X x : IHD = Example: C 4 H 8 O 2 : c= 4 h= 8 n= 0 o=2 x=0 IHD = =1 Draw two isomers for this molecule.
5 IHD from formula — Determine the IHD for these molecules: 1.C 17 H 21 NO 4 2.C 27 H 46 O 3.C 6 H 7 N 4.C 15 H 10 ClN 3 O 3 Using the ChemSpider RSC database look at the structure of these molecules and check to see if you calculations are correct. Using the ChemSpider RSC database look at the structure of these molecules and check to see if you calculations are correct.www.chemspider.com
6 ELECTROMAGNETIC SPECTRUM SL Chemistry Topic 11.3
7 **h = Plancks constant = 6.63 x 10 -34 J s E = energy of radiation **c = speed of light = 3.00 x 10 8 m s -1 = wavelength
9 Spectroscopy Visible and ultraviolet light: Electronic transitions between energy levels Gives information on the energy levels in an atom or molecule Basis for UV-vis spectroscopy
13 INFRARED SPECTROSCOPY SL Chemistry Topic 11.3
What Does Ihd Of 2 Mean
5/5wouldIHDtwo IHD
One degree of unsaturation is equivalent to 1 ring or 1 double bond . Two degrees of unsaturation is equivalent to 2 double bonds, 1 ring and 1 double bond, 2 rings, or 1 triple bond .
Likewise, what does an IHD of 0 mean? Each degree of unsaturation refers to a decrease in two hydrogens in the molecule, as a result of the presence of a pi bond or a ring. A degree of unsaturation of 0 means that the molecule follows the formula for an acyclic alkane .
Furthermore, what does the IHD tell you?
For hydrocarbons, the DBE tells us the number of rings and/or extra bonds in a non-saturated structure, which equals to the number of hydrogen pairs that are required to make the structure saturated, simply because joining two elements to form a ring or adding one extra bond in a structure reduces the need for
Can unsaturation degree be negative?
Can you ever have a negative – number of Degrees of Unsaturation? No, you can‘t ever have a –Negative number of Degrees of Unsaturation.
Don’t Miss: What Is Figure Ground Perception Psychology
Saturated And Unsaturated Compounds
In this post, we will talk about saturated and unsaturated compounds, the degresss of unsaturation , and the different ways of calculating it. Lets start with simple examples of;saturated and unsaturated compounds.
Suppose you are asked to identify the following molecules as saturated or unsaturated:
How do you distinguish these two types?
Simply put, the compounds that only have single bonds are saturated and the ones with a bond, which can be either a double or a triple bond are classified as unsaturated.
So, looking at these, we can identify compounds;A, D, E;as saturated and;B,;F as;unsaturated.
Compound C is a cycloalkane and even though there are no; bonds in it, it is considered to have a degree of unsaturation. The reason for this is the lack of the two hydrogens that are expected for the corresponding alkane with six carbons.
Lets go in order and;talk about what is called hydrogen deficiency index or degrees of unsaturation first.
How Do You Calculate Sodar
4.8/5SODARSODAR
For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons, Plus 2. Each Double Bond or Ring Reduces The Hydrogen Count By 2. Each Ring Or Double Bond Is Called A Degree Of Unsaturation Example: Benzene
Furthermore, what does IHD of 4 mean? IHD for C4H6 is. 2+262=2. This means it can have either one double bond and a ring such as or two double bonds such as CH2=CHCH=CH2 or CH2=C=CHCH3 or two rings , or one triple bond, such as CH3CCCH3.
Similarly, you may ask, how do you calculate HDI in chemistry?
The formula for an alkane is C_nH_. For a cycloalkane or an alkene, the formula is C_nH_2n. Each time you insert a double bond or a ring, you lose two H atoms. So, a double bond or ring means a deficiency of 2 H atoms.
What is the IHD formula?
A popular form of the formula is as follows: IHD = C + 1 + N/2 H/2 X/2. where C, N, H and X represent the number of carbon, nitrogen, hydrogen and halogen atoms, respectively.
Recommended Reading: Example Of Span Linear Algebra
Whats So Great About The Ihd Or Dus
The best thing about the IHD is that it is an easy equation to use that gives you useful informationabout the structure of an unknown compound. It literally takes a minute to do.;Calculating thedegrees of unsaturation is THE first task I recommend doing when you are faced with determiningthe structure of an unknown compound with a known molecular formula.
1 Before we get going,;see if you can apply the equation in the box above to;find the degrees ofunsaturation in;this;extremely well-known molecule whose formulae is given below.
C9H8O4
Calculating The Degree Of Unsaturation
If the molecular;formula is given, plug;in the numbers into this formula:
- C is the number;of carbons
- N is the number;of nitrogens
- X is the number;of halogens
- H is the number;of hydrogens
As stated before, a saturated molecule contains only single bonds and no rings. Another way of interpreting this is that;a saturated;molecule;has the maximum number of hydrogen atoms possible;to be;an acyclic alkane. Thus, the;number of hydrogens;can be represented by 2C+2, which is the general molecular representation of an alkane.;As an;example,;for;the molecular;formula;C3H4 the number of actual hydrogens needed;for the compound to be saturated is 8 . The compound needs;4 more hydrogens in order to be fully saturated . Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as;saturated.;;Hence, the DoB formula divides by 2. The formula subtracts the number of Xs because a halogen replaces a hydrogen in a compound. For instance, in chloroethane, C2H5Cl, there is one less hydrogen compared to ethane, C2H6.
For a compound to be saturated, there is one more hydrogen in a molecule when nitrogen is present. Therefore, we add the number of nitrogens . This can be seen with C3H9N compared to C3H8. Oxygen and sulfur;are not included in the formula because saturation is unaffected by these elements. As seen in alcohols, the same number of hydrogens;in ethanol,;C2H5OH, matches the number of hydrogens in ethane, C2H6.
| DoU |
You May Like: Introduction To Exponential Functions Common Core Algebra 1 Homework Answer Key
Index Of Hydrogen Deficiency
There is no simple way of predicting how many isomers a given molecular formula will yield, . Structures are different if they cannot be superimposed upon one another. Keep in mind that there is rotation about all single bonds not involved in a ring, but not about double bonds. Because all of the formulas that you will be dealing with are based on the \ atom, it may be useful to review the ways that \ can bond to itself and to other atoms. We will limit ourselves, for now, to the \ atom with four bonds. Below are the possible combinations of C having a total of four bonds.
In a hydrocarbon where all the C atoms have only single bonds and no rings are involved, the compound would have the maximum number of H atoms. If any of the bonds are replaced with double or triple bonds, or if rings are involved, there would be a deficiency of H atoms. By calculating the index of hydrogen deficiency , we can tell from the molecular formula whether and how many multiple bonds and rings are involved. IHD is also called the Degree of Unstaturation. This will help cut down the possibilities one has to consider in trying to come up with all the isomers of a given formula.
Here is a summary of how the index of hydrogen deficiency works.
- A double bond and ring each counts as one IHD.
- A triple bond counts as two IHD.
and \ stand for # of C and H respectively.)
| Example 1 |
|---|
Degree Of Unsaturation/index Of Hydrogen Deficiency
Now with lots functional groups introduced, the extent of constitutional isomers will be expanded a lot. To further explore the phenomena of constitutional isomers, we will need to understand the concept of Degree of Unsaturation;.
Lets compare three compounds first: pentane, 1-pentene and cyclopentane
The formula for pentane is C5H12. For a compound containing 5 carbons, the maximum number of hydrogens is 12, so the structure of pentane is saturated , or we can say that pentane has zero degree of unsaturation.
For 1-pentene C5H10, there are two less;hydrogens than the saturated level , which means the 1-pentene has one;degree of unsaturation. With a ring introduced, cyclopentane also has to sacrifice two;hydrogens, so cyclopentane also has one degree of unsaturation. The trend is that when a double bond , or a ring, is; involved in the structure, it leads to one degree of unsaturation of the compound.
Table 2.5 Summary of degree of unsaturation/IHD vs structure unit involved
The degree of unsaturation could be accumulated, and Table 2.5;summarizes the situations up to two degrees. As we can see, adding 1 ring or 1 bond contributes to one degree of unsaturation. Therefore, the essential meaning of degree of unsaturation is the number of rings plus ;bonds in a structure.
If the structure of a compound is available to us, the total degrees of unsaturation can simply be counted through inspecting the structure.
Example:
Solutions:
Recommended Reading: Segment And Angle Addition Worksheet
The Effect Of The Oxygen On Hdi
To determine the effect of an oxygen, lets compare the HDI of butane and the diethyl ether where the oxygen is placed in between the two ethyl groups:
What we see is that oxygen has no effect on the expected number of hydrogen atoms per carbon. The molecule still has 10 hydrogens for 4 carbons which corresponds to the general formula of alkanes, CnH2n+2.
So, whenever calculating the HDI for a molecule containing an oxygen atom, simply ignore it.
As an example, the degree of unsaturation for C7H14O2 is the same as C7H14 which corresponds to the CnH2ngeneral formula a with one degree of unsaturation.
What Is Meant By Sigma And Pi Bond
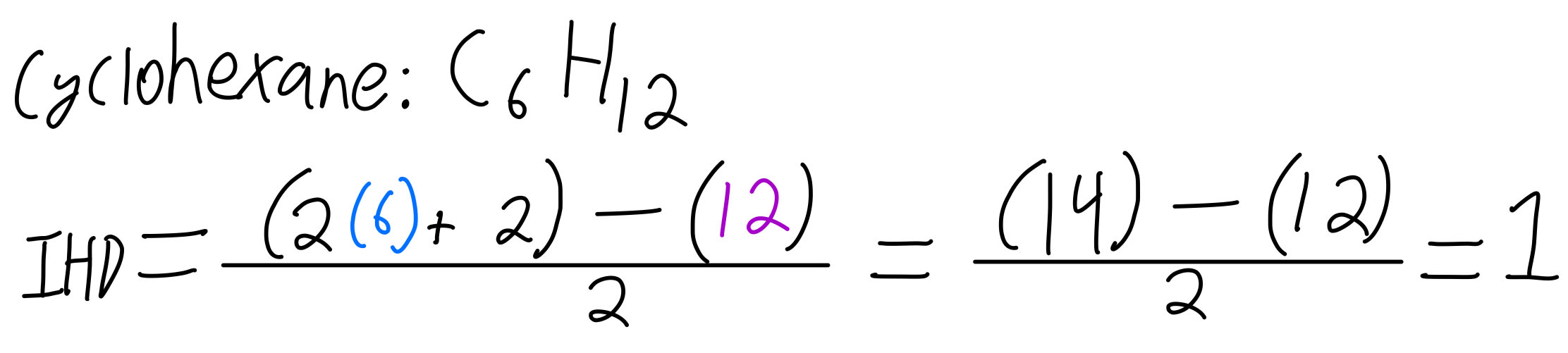
Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlap of two atomic orbitals.
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
How To Calculate The Degree Of Unsaturation
In many cases, it is quite straightforward to determine the degree of unsaturation based on the number of multiple bonds and rings present in the molecule:
Lets pay attention to one key factor here: For all of them, we had the Lewis structure provided to us and we only needed to visually assess the unsaturation. However, it is important to have a formula to do this because often only the formula of the molecule is given and the structure is unknown.
For example, you may need to determine the HDI for C6H10ClNO2.
The structure is determined based on different techniques such as IR and NMR spectroscopies and having the degree of saturation in hand makes the process much easier.
There are two ways to determine the HDI:
- ;Using a formula. There are different formulas for this but you can remember one and go with that.
Before determining the HDI of C6H10ClNO2, lets talk about the effect of each heteroatom on the number of expected Hs separately, starting with halogens.
For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons Plus 2
You should be able to see that for a hydrocarbon with no rings or double bonds, the number of hydrogens is equal to twice the number of carbons, plus 2.
So for a molecule like dodecane wed expect to see + 2 = 26 hydrogens.
Now: what happens to the molecular formula when we add a double bond to the molecule?
Recommended Reading: Solving Age Word Problems
What Does It Mean For A Compound To Be Saturated
A molecule that has the maximum number of hydrogen atoms is said to be saturated. A saturated alkanes molecular formula is as follows: CnH2n+2. For example, hexane is saturated but cyclohexane is not. Hexane and cyclohexane have the exact same number of carbons, but cyclohexane actually has two fewer hydrogens than does hexane.;
Each Ring Or Double Bond Is Called A Degree Of Unsaturation
Therefore, each ring introduces a degree of unsaturation into the molecule.;
You might ask: what if we have a molecule with rings and multiple bonds?;See for yourself.
The effect is additive. That is, the degrees of unsaturation is the;sum of the number of double bonds and rings.
Note that it doesnt tell you how many double bonds are present or how many rings are present. It merely tells you their sum.
Also Check: What Is The Molecular Geometry Of Ccl4
Determining Degrees Of Unsaturation From A Molecular Formula:
On an exam, you might be given a prompt like this one: Please provide the degree of unsaturation for each of the following compounds. Some of those might be molecular formulas like C6H14;or C6H12. For this type of problem, its easiest to start with a nice equation. In it, IHD will stand for index of hydrogen deficiency .
Degrees of Unsaturation general formula
So, now that weve got the formula, lets apply it to our friends hexane and cyclohexane before testing other molecules.;
IHD hexane formula
How Do You Calculate Degrees Of Unsaturation In Organic Chemistry
4.4/5CalculatingDegree of UnsaturationDegrees of unsaturation
Each row corresponds to a different combination. One degree of unsaturation is equivalent to 1 ring or 1 double bond . Two degrees of unsaturation is equivalent to 2 double bonds, 1 ring and 1 double bond, 2 rings, or 1 triple bond .
One may also ask, how are DBES calculated? The DBE number can be calculated from the formula using the following equation: DBE = UN = PBoR = C – + +1, where: C = number of carbon atoms, H = number of hydrogen and halogen atoms, and N = number of nitrogen atoms. One DBE = one ring or one double bond.
Similarly, what does degree of unsaturation of 4 mean?
For A Hydrocarbon With No Rings Or Double Bonds The Number Of Hydrogens Is Equal To Twice The Number Of Carbons, Plus 2. Each Double Bond or Ring Reduces The Hydrogen Count By 2. Each Ring Or Double Bond Is Called A Degree Of Unsaturation Example: Benzene
What does an IHD of 2 mean?
2. This means it can have either one double bond or one ring. It cannot have a triple bond. Since you cannot form a ring with only two C’s, it must have a double bond. Example 2: IHD for C4H6 is.
Don’t Miss: Segment Addition Postulate Answers
Each Double Bond Or Ring Reduces The Hydrogen Count By 2
You should be able to see that each multiple bond the number of hydrogens in the formula decreases by two. Ethyne has two fewer hydrogens than ethene , which has two fewer hydrogens than ethane .
Hydrocarbons containing ; bonds are often called unsaturated hydrocarbons. They can be treated with hydrogen to give the corresponding alkane with no ; bonds, which is then said to be saturated with hydrogen. .
Since every pi bond results in a loss of 2 hydrogens from the molecular formula, we refer to this to as a degree of unsaturation.
Lets turn our attention to cyclic compounds. Do you notice a similar effect?
You should! Every ring in the molecule decreases the number of hydrogens by two.