Kinetics Techniques In Combination With Uniform Supersonic Flows
In a CRESU apparatus, the reaction to be investigated has to be initiated for the study. When working on radicals, the most common way to do it is using pulsed laser photolysis , typically employing high-power UV pulsed excimer lasers operating at 193 nm or 248 nm , or the fourth harmonic of an Nd:YAG laser at 266 nm.
In the CRESU experiment, the pseudo-first-order-technique is used if possible.34 This technique allows the measurement of a second-order rate constant with multiple first-order experiments, as described previously. A few µs after the photolysis pulse, the tuneable laser pulse excites the tracked species to probe its concentration. This causes fluorescence, which is collected by a photomultiplier tube that converts a light intensity into a measurable current, in a linear fashion. The resulting current is converted into a voltage which is integrated and digitized, and the integrated value plotted against the time delay. The pair of PLP and LIF laser pulses are repeated for a different time delay. With sufficient pairs of PLP-LIF pulses, a decay curve is obtained, which should be single exponential in the case of pseudo-first-order kinetics. With multiple pseudo-first-order experiments, it is possible to obtain the second-order rate constant of the reaction measured.
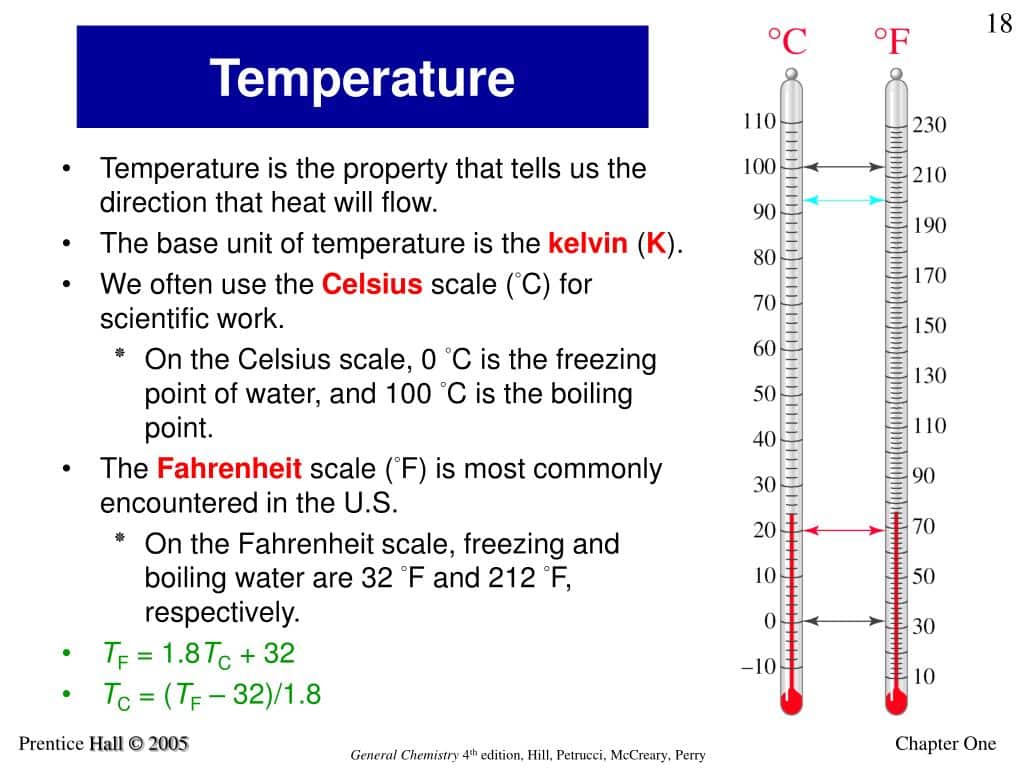
Room Temperature Of A Typical Home
According to the American Heritage Dictionary of the English Language, room temperature is 2022 °C . The Oxford English Dictionary states room temperature is around 20 °C . Merriam-Webster defines a temperature range of 15 to 25 °C as suitable for long term human occupancy and laboratory experimentation.
However, room temperature tends to be cooler in winter and warmer in summer, to accommodate seasonal clothing. Also, studies indicate women often prefer warmer temperatures than men. The World Health Organization recommends a minimum room temperature of 20 °C for infants, children, the elderly, and people who are ill.
Radicalradical Reactions: O + Oh O2 + H
A novel VUV co-photolysis technique was chosen to generate the necessary concentrations of ground-state atomic oxygen and the low concentrations of OH radicals. Using Laval nozzles designed to work with N2 buffer gas at temperatures down to 39 K, Carty et al.36 introduced a mixture of known concentrations of molecular oxygen and trace quantities of water vapour into the supersonic flow. An F2 excimer laser operating at 157 nm was then used to produce oxygen atoms by photolysis of molecular oxygen, and OH radicals via photolysis of water. At each O2 concentration used, the fluence of the VUV laser was carefully measured in situ and this, coupled with knowledge of the O2 photodissociation cross-section at 157 nm, enabled the calculation of the O atom concentration. The photodissociation of O2 at 157 nm produces both O and O atoms, and so the use of N2 as a buffer gas was essential in order to rapidly relax the excited-state O atoms to O. This co-photolysis thus created the necessary pseudo-first-order conditions and the subsequent decay of OH radical concentration was followed by LIF using a second pulsed tuneable dye laser operating at 282 nm.
Upper panel:Lower panel:ket al.J. Chem. Phys.
Recommended Reading: Why Do We Dream Psychology
Kinetic Energy And Temperature
According to the kinetic-molecular hypothesis, a substance’s temperature is proportional to the average kinetic energy of its particles. When a substance is heated, some of the energy absorbed is kept inside the particles, while another energy accelerates particle motion. This is manifested as a rise in the material’s temperature.
When studying kinetic energy in gas molecules and its relationship with temperature, we generally define the term average kinetic energy.
What Is Standard Temperature And Pressure
Standard temperature and pressure refers to the nominal conditions in the atmosphere at sea level. These conditions are 0 degrees Celsius and 1 atmosphere of pressure. The STP value is important to physicists, chemists, engineers, pilots and navigators, among others.
Standard conditions for temperature and pressure refers to a specific pressure and temperature used to report on the properties of matter. STP values are commonly used in experiments involving gases.
In the past, the International Union of Pure and Applied Chemistry defined STP as the following:
- Temperature: 0 degrees Celsius
- Pressure: 1 atm .
This definition is now discontinued. Nonetheless, these conditions are still commonly used to define the volume term normal cubic meter. Since 1982, IUPAC has applied a more stringent definition of STP:
- Temperature: 0 degrees Celsius
- Absolute pressure: 100,000 pascals or 105 Pa .
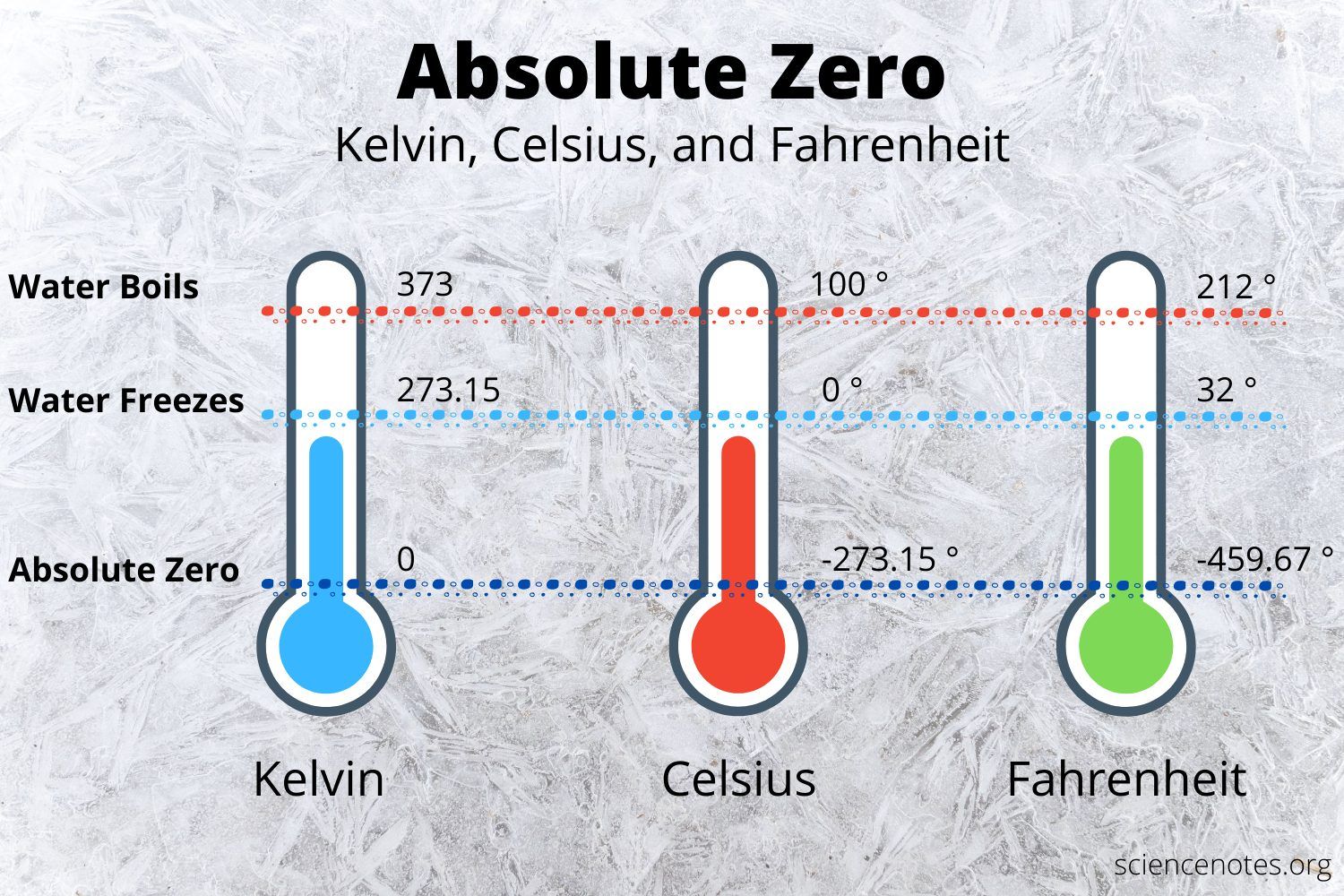
Thus, standard temperature is defined as 0 degrees Celsius, which translates to 32 degrees Fahrenheit or 273.15 degrees Kelvin. This is essentially the freezing point of pure water at sea level in air at standard pressure. The National Institute of Standards and Technology defines STP differently as absolute pressure of 1 atm and 20 degrees Celsius .
Don’t Miss: How To Do Powers In Math
Bodies In Thermodynamic Equilibrium
For experimental physics, hotness means that, when comparing any two given bodies in their respective separate thermodynamic equilibria, any two suitably given empirical thermometers with numerical scale readings will agree as to which is the hotter of the two given bodies, or that they have the same temperature. This does not require the two thermometers to have a linear relation between their numerical scale readings, but it does require that the relation between their numerical readings shall be strictly monotonic. A definite sense of greater hotness can be had, independently of calorimetry, of thermodynamics, and of properties of particular materials, from Wien’s displacement law of thermal radiation: the temperature of a bath of thermal radiation is proportional, by a universal constant, to the frequency of the maximum of its frequency spectrum this frequency is always positive, but can have values that tend to zero. Thermal radiation is initially defined for a cavity in thermodynamic equilibrium. These physical facts justify a mathematical statement that hotness exists on an ordered one-dimensional manifold. This is a fundamental character of temperature and thermometers for bodies in their own thermodynamic equilibrium.
Discuss Student Observations And What May Have Caused The Temperature Of The Metal Washers And Water To Change
Ask students:
- How did the temperature of the washers and water change in both parts of the activity?
- Based on their data, students should realize that the temperature of both the washers and water changed.
- Knowing what you do about heating and cooling atoms and molecules, why do you think the temperature changed?
- If necessary, guide students thinking about why the temperature of each changed by asking them which were probably moving faster, the atoms in the metal washers or the molecules in the water. Tell students that the molecular model animation you will show next will show them why the temperature of both changed.
Don’t Miss: Do Not Open This Math Book
The Common Benchmarks Of Temperature In Chemistry
The study of chemistry wonât be precise if there arenât any benchmarks for certain reactions, chemical behaviours and properties. In most cases, the IUPAC standard conditions are used to define certain characteristics of chemicals.
The standard conditions use temperature and pressure as the parameters:
Effect Of Temperature On Rate Of Reaction
Reaction Kinetics deals with the Study of Rates of Chemical Reactions or Processes. It is one of the most advanced studies in the field of physical chemistry. The information about these factors affecting the rate of a chemical reaction help engineers and scientists to economically scale up the reactions to industrial scale in various industries. It is therefore very important for us to know how the rate of a chemical reaction gets affected.
Also Check: Unit 1 Geometry Basics Homework 4
Radicalmolecule Reactionso + Alkenes
One of the most surprising results to come out of the early CRESU work on neutralneutral reactions was a study on the reactions of the CN radical with a number of hydrocarbons, both unsaturated and saturated .10 Not only were the rate constants for these radicalmolecule reactions all found to be fast at very low temperatures , but the rate constant for the CN + C2H6 reaction was found to display a minimum with Arrhenius-type behaviour at higher temperatures, and barrierless-type behaviour at lower temperatures. This was understood in terms of the transient formation of a pre-reactive complex,11 and subsequent passage over a barely submerged, but tight barrier,12 corresponding to case c in Figure 1.2.
Science317
What Is Room Temperature
Room temperature is defined as the thermometer reading of a room. Ideally, it is the temperature at which people feel comfortable wearing ordinary clothing. For most people, its either the usual temperature of their house or the temperature they set the thermostat. In science, room temperature is often defined. Here is a look at the different values of room temperature.
Don’t Miss: What Is Climate In Geography
Product Branching Studies At Low Temperaturesc2h + Butenes
The study of the kinetics of C2H reactions for astrochemical interest began very early, since it was one of the first species detected in the interstellar medium as early as 1974,75 on Titan in 1991,76 and more recently in star formation regions.77 Following this interest, numerous studies of the reactivity of C2H and the products of these reactions have been carried out.78
This section presents a set of experiments79 that were performed on the Laval machine, a pulsed Laval nozzle system in use at the Advanced Light Source synchrotron .80 This experimental setup samples the uniform flow through a pinhole in an airfoil, followed by synchrotron VUV ionization, and quadrupole mass discrimination. The main advantage of this setup is the low gas consumption and the very high resolution in product discrimination. The study did not evaluate a temperature change effect, and was focused on the study of C2H radical colliding with butene isomers: 1-butene, 2-butene , and isobutene and reaction products, at 79 K. The reaction C2H + 1-butene is treated here as example. The process does not differ for other reactions in the study.
First, a mass spectrum at high ionization energy is obtained with only 1-butene in the flow, to study its fragments. This is the spectrum A in Figure 1.17. Products of dissociation were identical to previous work.81
Have Students Try One Or More Extensions And Use Conduction To Explain These Common Phenomena
Compare the actual temperature and how the temperature feels for different objects in the room.
Ask students:
- Touch the metal part of your chair or desk leg and then touch the cover of a textbook. Do these surfaces feel like they are the same or a different temperature?
- They should feel different.
- Why does the metal feel colder even though it is the same temperature as the cardboard?
- Tell students that even though the metal feels colder, the metal and the cardboard are actually the same temperature. If students dont believe this, they can use a thermometer to take the temperature of metal and cardboard in the room. After being in the same room with the same air temperature, both surfaces should be at the same temperature.
Tell students to watch the motion of the molecules in the metal, cardboard, and in the finger.
Explain that the molecules in your finger are moving faster than the molecules in the room-temperature metal. Therefore the energy from your finger is transferred to the metal. Because metal is a good conductor, the energy is transferred away from the surface through the metal. The molecules in your skin slow down as your finger continues to lose energy to the metal, so your finger feels cooler.
You May Like: What Is Biosphere In Biology
The Meaning Of Temperature In Chemistry
Temperature measures how much kinetic energy molecules or atoms in a substance have.
It can also represent the radiant energy of an electromagnetic wave, as demonstrated by microwaves.
In chemistry, when energy is applied to a substance/system or when energy is released by a substance, its temperature rises. For example, the reaction of metal sodium with water is extremely exothermic and usually accompanied by a flame as the hydrogen is liberated from the water.
2Na + 2H2O â 2NaOH + H2
In this case, there is no energy input necessary to start the reaction. The heat energy comes from the breaking of the covalent bonds between the oxygen atom and the hydrogen atom in the water molecule.
Conversely, when energy is taken away from a substance/system, or if energy is absorbed by a substance/system, the temperature decreases. Photosynthesis is a good example of an endothermic reaction. Although itâs a complex process that involves several steps and chemical intermediates, as well as chlorophyll, the chemical reaction can be summarised as follows:
In photosynthesis, glucose is produced with the help of energy input from the sun or any other artificial light source. Hence, the reaction is considered endothermic because it absorbs energy.
What Happens If You Store A Battery In A Cold Environment
Did you ever notice that your phone battery is drained much quicker during a cold day when temperatures are below 5ºC ?
This happens because, in cold temperatures, the batterys electrolyte becomes less conductive, which leads to an increase in the batterys internal resistance.
Therefore, the voltage that pressure that pushes electrons through a circuit decreases, along with your batterys power output .
However, when the battery is not being used, no electrons are pushed through a circuit.
Nonetheless, if you measure the voltage between the battery terminals, youll read the open-circuit voltage. This open-circuit voltage is the potential difference between the terminals, and its associated with the batterys internal resistance.
The potential difference decreases if the internal resistance increases. This can be harmful to the battery because the open circuit voltageis proportional to the batterys state of charge.
In turn, storing your battery at extreme states of charge either too low or too high may lead to degradation of materials or loss of battery efficiency.
Think of it this way: if a lithium battery is stored at a high state of charge, too many lithium atoms will be ready to let go of their valence electrons . Therefore, the battery will be in a stressed state.
Conversely, if you store it at a state of charge of around 40% or 50%, the chemical reactions will be at equilibrium so that the battery wont be stressed.
In Summary
Recommended Reading: What Is Token Economy In Psychology
Curie Temperature In Chemistry
This benchmark is named after Nobel Prize winner, Marie Curie. Otherwise known as the Curie point, the Curie temperature is defined by the temperature threshold at which magnetic materials significantly change their magnetic properties. It varies from one material to another, but the Curie temperature for the mineral magnetite is about 570°C .
The Need For Standard Temperature And Pressure
Both temperature and air pressure vary from one place to another. To test, compare and document chemical and physical processes where temperature and pressure play a role, including data centers or anywhere computers are used, a standard reference of both is required. Certain properties of matter also vary with changes in temperature or pressure. These include the following:
- melting point
- boiling point
A reference value of temperature and pressure accommodates comparisons and measurements of processes. It also enables better understanding and comparison of various properties of matter. STP provides such a reference.
STP conditions are important to calculate and express fluid flow rates and the volumes of liquids and gases when standard state conditions are applied. These properties are also highly dependent on temperature and pressure conditions and changes. Adopting and stating standard conditions enable similar experiments to occur in similar laboratory conditions and to generate similar and comparable results. It also makes it easier to compare different measurements for gases, such as the moles of gas in a given volume.
You May Like: Are Men Biologically Stronger Than Women
The Science Of Cryogenics
Cryogenics is branch of physics that incorporates the production and effects of substances at temperatures ranging between -150ºC to -273°C. In the late 1870’s, cryogenic science began with the cooling of gaseous oxygen. At -183ºC, O2 condensed from a clear gas into a blue liquid. Changing the state of oxygen enables it to be transported easier. Both the aircraft and medical industries rely on the liquid form of this molecule to provide breathable oxygen for pilots, travelers, and patients.
Besides oxygen, other gases have been condensed as well. These permanent gases, meaning these substances normally exist in the gaseous state, include nitrogen , hydrogen , chlorine , and helium . By manipulating pressure and/or temperature, many chemists and physicists have attempted to produce the lowest, theoretical temperature possible. At absolute zero , the particles of a material have a minimum kinetic energy. Click on this video to determine if scientists can ever reach absolute zero in their laboratories.
Investigating a Cryogenic material: \
Liquid N2 is also utilized to freeze biological samples. Fertility clinics freeze semen, eggs, and embryos that can be used at a later date for couples who chose to undergo InVitro Fertilization . Umbilical cord blood and stem cells can be preserved in liquid nitrogen for future applications.
References
A Quick Review On Battery Related Terms
Before we deepen our discussion, heres a review of a few basic concepts that should help you better understand whats to come in the article.
Voltage: you can think of voltage as the pressure that pushes the electrons through a circuit.
Open circuit voltage: is the voltage between the batterys terminals when the battery is not connected to a load .
Self-discharge: the chemical reactions inside the batterys cell occur without any connection between the electrodes or an external circuit. This phenomenon reduces the batterys stored capacity even when not in use.
Electrolyte: the electrolyte sits between the anode and the cathode and allows ions to move between these electrodes thus, chemical energy is converted into electric energy.
State of charge : expresses the present capacity of the battery relative to its total capacity.
Lithium Iron Phosphate Sealed Lead-Acid
Read Also: How Much Do Physics Professors Make