Common Acid And Anion Names
Acids are compounds containing an ionizable proton , since an acid is a proton donor . The polyatomic anions derived from acids are named by dropping the-ic suffix from the acid name and adding the-ate suffix, respectively. Compounds containing polyatomic anions are named using the Type I or Type II naming systems described above. For example, the sodium salt of nitric acid is sodium nitrate . If you know the acid formula you will alwaysget the correct anion formula and its charge, since the charge is equal to the number of ionizable hydrogen atoms in the acid, and is always negative. For example, for sulfuric acid , the anion is sulfate with a -2 charge.
Acids which do not contain oxygen are named by adding the hydro- prefix to the root name of the element, followed by the -ic suffix. HCl ishydrochloric acid, H2S ishydrosulfuric acid, and HF is hydrofluoric acid . Anions of these acids, which contain a single element , are named as a regular non-metal anion .
| Acid |
|---|
Introduction To Chemistry Prefixes
9: nona
10: deca
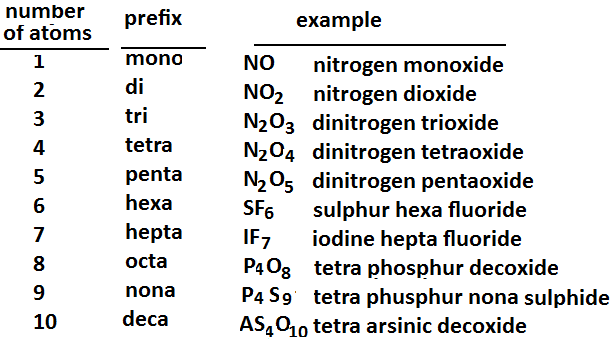
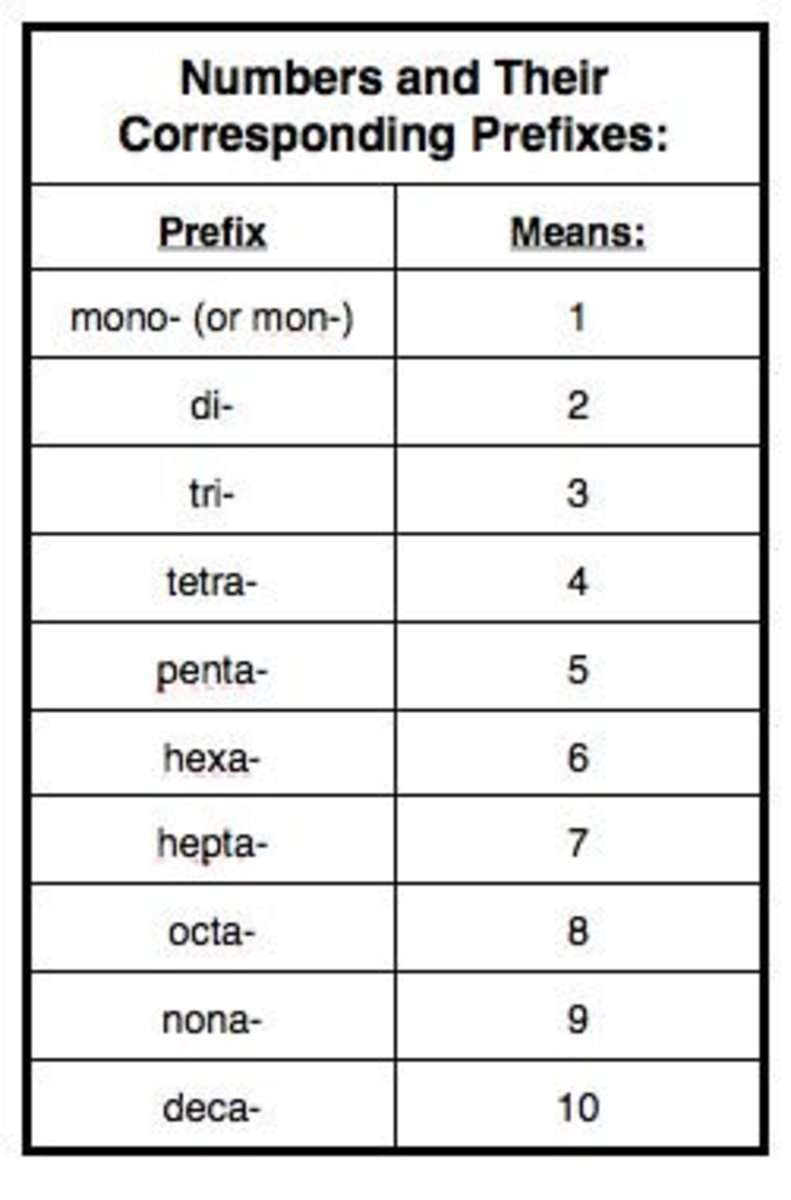
The above list shows the 10 most basic chemistry prefixes for naming compounds, which come from Greek. These prefixes can be used to name just about any compound. With a little bit of practice, naming compounds will become easier and easier! Dont get frustrated with yourself if you dont understand it right away.
Explanation Of Naming Compounds
The naming system is used by determining the number of each atom in the compound. For example, a compound that has 5 atoms of a particular element would have the penta prefix before that element in the compounds name. Once you have determined each prefix, you need to add the ide suffix if the second name in the compound is an element .
Also Check: Answers To Holt Mcdougal Geometry
What Are All The Prefixes In Chemistry
When naming molecular compounds prefixes are used to dictate the number of a given element present in the compound. mono- indicates one, di- indicates two, tri- is three, tetra- is four, penta- is five, and hexa- is six, hepta- is seven, octo- is eight, nona- is nine, and deca is ten.
Read Also: Holt Mcdougal Geometry Answers And Work
How To Name Molecular Compounds: Rules And Examples
In chemistry, the naming of molecular compounds helps in the determination of the number of atoms and the nature of a compound. A molecular compound is formed when two or more atoms are bonded together in a fixed proportion.
Since ancient times, elements and compounds have been represented by symbols. John Dalton had a unique way of representing the elements and compounds of his time.
The representation symbols of some chemical elements and compounds by John Dalton are given below.
Later on, the way of naming elements and compounds got changed from symbols to writings. The works of 19th-century Swedish chemist Jason Jakob Berzelius led to this invention. He started a system of naming elements via letters. These letters would most probably be the first letters of the names of elements. According to Berzelius, hydrogen, oxygen, nitrogen, carbon, phosphorous, and sulfur were named H, O, N, C, P, S respectively.
Read Also: Beth Thomas Documentary
The Stock Method Of Naming
An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element. For example, K+1 is called the potassium ion, just as K is called the potassium atom. The anion is named by taking the elemental name, removing the ending, and adding -ide. For example, F-1 is called fluoride, for the elemental name, fluorine. The -ine was removed and replaced with -ide. To name a compound, the cation name and the anion named are added together. For example, NaF is also known as sodium fluoride.
If either the cation or the anion was a polyatomic ion, the polyatomic ion name is used in the name of the overall compound. The polyatomic ion name stays the same. For example, Ca2 is called calcium nitrate.
For cations that take on multiple charges , the charge is written using Roman numerals in parentheses immediately following the element name. For example, Cu2 is copper nitrate, because the charge of two nitrate ions is 2 = -2. Since the net charge of the ionic compound must be zero, the Cu ion has a 2+ charge. This compound is therefore, copper nitrate. The Roman numerals in fact show the oxidation number, but in simple ionic compounds this will always be the same as the metals ionic charge.
Turn Oa And Oo Into A Single O
Sometimes, while naming the chemical compounds, a problem arises when compounds with ao and oo are written with -ide name. Conventionally, they are converted into a single o. For example, the ao in the dichlorine hept-ao-xide . This ao in the Cl2O7 can be converted into o. As the result, the correct name of this compound would be dichlorine hept-o-xide.
Examples of molecular compounds with their names:
- Carbon monoxide CO
- Silicon tetraiodide SiI4, etc
Read Also: How To Calculate Half Life Chemistry
Ionic Compounds Containing Polyatomic Ions
A special type of ionic compound is exemplified by ammonium nitrate , which contains two polyatomic ions, NH4+ and NO3. As the name suggests, a polyatomic ion is a charged entity composed of several atoms bound together. Polyatomic ions have special names that are used in the nomenclature of the compounds containing them.
| Common polyatomic ions | |
|---|---|
| *Bisulfate and **bicarbonate are widely used common names for hydrogen sulfate and hydrogen carbonate, respectively. | |
| O22 | peroxide |
Several series of polyatomic anions exist that contain an atom of a given element in combination with different numbers of oxygen atoms. Such anions are called oxy anions. When the series contains only two members, the name of the ion with fewer oxygen atoms ends in -ite, and the name of the other ion ends in -ate. For example, SO32 is called sulfite and SO42 is called sulfate. In those cases where more than two oxy anions constitute the series, hypo- and per- are used as prefixes to name the members of the series with the smallest and the largest number of oxygen atoms, respectively. The chlorine-containing oxy anions provide an example:
| ClO | |
| ClO4 | perchlorate |
Iupac Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry . It is published in the Nomenclature of Organic Chemistry . Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially shorter and clearer, and so preferred. These non-systematic names are often derived from an original source of the compound. Also, very long names may be less clear than structural formulas.
You May Like: My Hrw Com Algebra 1
You May Like: Geometry Basics Segment Addition Postulate Worksheet Answer Key
Rules For Naming Molecular Compounds
- Write the name for both elements.
- Change the ending of the second element to ide.
- Place prefixes in front of each element based on the number of atoms present.
- The prefix ‘mono’ is only used on the second non-metal in the chemical formula.
- There shouldn’t be two vowels in a row. For example, when you have ‘mono’ in front of ‘oxide’ it is written ‘monoxide’, not ‘monooxide’.
Why Is Nomenclature Important What Is The Purpose Of Nomenclature
There are two objectives of using nomenclature in chemistry:
- To make sure that a spoken or written chemical name does not contain any ambiguity regarding the chemical compound the name is referring towards. It is important that each chemical name points towards a single substance.
- To ascertain that each substance has one name only
- To help the chemists communicate with their peers easily.
Emma
Don’t Miss: Geometry Of Ccl4
What Are The General Rules For Nomenclature What Are Nomenclature Rules
Scientists employ nomenclature to name compounds clearly in chemistry. Ionic and molecular compounds are named using distinct methods. Nomenclature in chemistry refers to a set of rules to generate systematic names of compounds. The nomenclature which is used by the chemists and scientists worldwide is created and developed by the IUPAC .
The Old Classic Or Common Way Of Naming
Names of some ionic compounds: Common, or trivial, names of compounds are sometimes used in informal conversations between chemists, especially older chemists. Systematic names are formal names that are always used in print.
Since some metallic elements form cations that have different positive charges, the names of ionic compounds derived from these elements must contain some indication of the cation charge. The older method uses the suffixes -ous and -ic to denote the lower and higher charges, respectively. In the cases of iron and copper, the Latin names of the elements are used . This system is still used, although it has been officially supplanted by the more precise, if slightly cumbersome, Stock system. In both systems, the name of the anion ends in -ide.
Naming Compounds Part 1 YouTube: This video explains how to name covalent and ionic compounds.
Also Check: Ccl4lewis Structure
What Does The Per Mean In Chemistry
ChemistryprefixPart 1Writing Chemical Formulas of Covalent Compounds
Rules For Naming Molecular Compounds:
Generally, the more electropositive atom is written first, followed by the more electronegative atom with an appropriate suffix. For example, H2O can be called dihydrogen monoxide . Organic molecules do not follow this rule.
Don’t Miss: How To Find Half Life
For The Second Element End With The
This step involves the addition of -ide at the end of elements, such as sulfide , oxide , etc.
The second element ends with the -ide name. For example, in the case of dinitrogen trioxide . The second element is oxygen. At the start of oxygen, is used as a prefix, and at the end of oxygen, is used. For example, Dinitrogen pentox.
Prefixes Of Base Units By Factors Of Ten
Metric or SI units are based on units of ten. Very large or very small numbers are easier to work with when you can replace any scientific notation with a name or word. The metric unit prefixes are short words that indicate a multiple or fraction of a unit. The prefixes are the same no matter what the unit is, so decimeter means 1/10th of a meter and deciliter means 1/10th of a liter, while kilogram means 1000 grams and kilometer means 1000 meters.
Decimal-based prefixes have been used in all forms of the metric system, dating back to the 1790s. The prefixes used today have been standardized from 1960 to 1991 by the International Bureau of Weights and Measures for use in the metric system and the International System of Units .
Recommended Reading: Exponential Modeling With Percent Growth And Decay Common Core Algebra 2 Homework
What Are The Three Types Of Compounds
We all know that a chemical element has one type of atom only. When a substance contains more than one kind of atom, then we say that it is a compound. In other words, we can say that a compound refers to a substance in which two or more atoms are bonded with each other.
Millions of compounds exist and all fall in the following three broad categories:
1) Ionic Compounds These compounds are made up of ions. Ions are charged particles that are made when an atom gains or loses electrons. There are two types of ions: cation and anion. A cation is a positively charged ion and the anion is a negatively charged ion. These compounds are generally formed by a reaction between a metal and a nonmetal. To determine how to name these compounds, see the rules for naming ionic compounds in the previous section. 2) Molecular or Covalent Compounds They are formed when elements of the compound share electrons in a covalent bond to make up a molecule. These compounds are formed by the reaction between two nonmetals. 3) Acids Acids are compounds that contain hydrogen. It is easy to recognize acids as they contain hydrogen and anion. For instance, is named as nitric acid and is named as sulphuric acid.
What Are The Prefixes In Organic Chemistry
The ones that students confuse all the time are below:
Methane 1 carbon compound
For compounds with 5-10 carbons, add the Greek prefix to -ane.
For example,
Get a free answer to a quick problem. Most questions answered within 4 hours.
OR
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
Read Also: Percent Error Calculation Chemistry
How Do You Name A Company
Here are 12 helpful suggestions on how to come up with a winning name for your business:
Prefixes For Molecular Compounds

Prefixes in molecular compounds are decided by the number of atoms of each element in the compound. The first step is to count the number of each element. Then, assign a prefix based on the list at the beginning of this article . Put the two elements together, and dont forget the ide on the second element. To make life easier, you dont need to include the prefix mono for the first element of the two. For example, NO2 would be called nitrogen dioxide, not mononitrogen dioxide. Remember that this rule only applies to the first element of the two.
Also Check: Hawkes Learning Systems Statistics Answer Key
How Do You Know Whether To Use ‘ide’ Or ‘ate’ When Naming A Compound
-ide is used for non-metal compounds generally. For example, Chlorine forms a chloride ion, so NaCl is Sodium Chloride. -ate and -ite are commonly used for polyatomic ions of Oxygen. -ate is used for the ion that has the largest number of Oxygen atoms. The -ite would be used for the ion with the smaller. NO2 and NO3 are known as Nitrite and Nitrate respectively. Nitrite has a smaller number of oxygen atoms so when added to an element it will be _ Nitrite. On the other than, Nitrate has a larger number of Oxygen atoms so when added to an element it is _ Nitrate Share your tips and advice for learning the names of chemical compounds in the comments.
Key Concepts And Summary
Chemists use nomenclature rules to clearly name compounds. Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to ide. For example, K2O is called potassium oxide. If the metal can form ions with different charges, a Roman numeral in parentheses follows the name of the metal to specify its charge. Thus, FeCl2 is iron chloride and FeCl3 is iron chloride. Some compounds contain polyatomic ions the names of common polyatomic ions should be memorized. Molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. Binary acids are named using the prefix hydro-, changing the ide suffix to ic, and adding acid HCl is hydrochloric acid. Oxyacids are named by changing the ending of the anion to ic, and adding acid H2CO3 is carbonic acid.
Read Also: Lewis Structures And Molecular Geometry Lab Answers
How Do You Name Compounds In Chemistry
The elements that are joined together through chemical bonds are known as chemical compounds. The chemical bonds between the compounds are strong enough to make them act like a single substance. Do you know how many compounds are there? The answer is that there are more than 350,000 chemical compounds that are registered for use and production. You can easily search the list of compounds online. The properties of compounds are different than those of the elements that were used to make those compounds. Now, the question arises how these compounds are named in chemistry? The answer is simple. There is a standard method of naming chemical compounds that is employed by all the scientists worldwide.