Fish And Hypertonic Solutions
Saltwater tends to be hypertonic to the fish that live in it. Since they require a lot of surface area of their bodies to contact water they lose a lot of water through osmosis of the gill linings. Many fish have evolved to get around this problem though they drink large amounts of salt water and excrete any excess salt from their bodies.
Filtration In The Kidneys
Basic biology tells us hydration is essential to body function. The kidneys, in particular, depend on hydration to effectively remove excess minerals and waste, which mix with liquids to form a solution, from the body.
Under normal conditions, liquids move through the kidneys, which filter excess minerals and waste. These excess materials depend on liquids to both move through and exit the body. In fact, without water to propel them through the bladder and out the urethra, these solutes build up in the kidneys to cause kidney stones or, in extreme cases, kidney failure.
When the excess minerals and waste in the kidney is greater than the amount of liquid, the solution in the interior of the kidneys is said to be hypertonic to the solution of unfiltered liquids passing through. Because there are not enough liquids to move them out of the body, excess minerals and waste build up, and can form stones. If these stones go untreated, they can congest the kidneys, for lack of a better term, and lead to kidney failure.
What Does Isotonic Mean In Biology
4.4/5isotonicin-depth answer
Isotonic solutions contain an electrolyte balance similar to plasma in the bloodstream. When an isotonic solution is administered, the fluid volume of the patient is increased without a fluid shift. Common examples of isotonic solutions are 0.9% normal saline and lactated ringers.
Also, what does isotonic and hypertonic mean? If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. In an isotonic environment, the relative concentrations of solute and water are equal on both sides of the membrane. When a cell is placed in a hypotonic environment, water will enter the cell, and the cell will swell.
Keeping this in consideration, what does hypotonic mean in biology?
A hypotonic solution is any solution that has a lower osmotic pressure than another solution. In the biological fields, this generally refers to a solution that has less solute and more water than another solution.
What is the difference between isotonic and hypotonic?
Hypotonic refers to a lesser concentration. In biology, a hypotonic solution has a lower concentration of solutes outside the cell than inside the cell. An isotonic solution is one in which its effective osmole concentration is the same as the solute concentration of a cell.
You May Like: Geometry Escape Challenge A Answer Key
When A Cell Is Kept In Hypertonic Solution It Becomes
Solution. In a hypertonic solution, the solution outside the cell has higher solute concentration than the fluids inside the cell. Therefore, water flows out from the plant cell due to exosmosis. The cytoplasm shrinks and the plasma membrane withdraws away from the cell wall and this the cell becomes flaccid.
Why Dont Skin Cells Burst When You Take A Bath
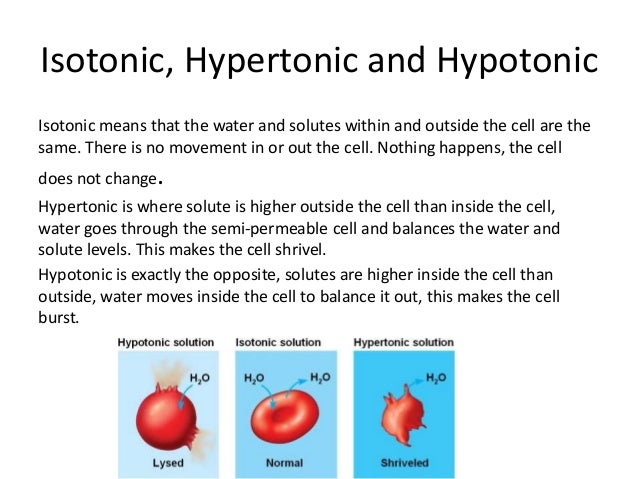
Why don t skin cells burst when you take a bath? As a human being, though, your skin is waterproofed with oils that are secreted by the sebaceous glands in the hair follicles, and so entry of water across the organ, by osmosis, is reduced. The control of water-levels in the body is a part of the process of HOMEOSTASIS.
Recommended Reading: Are Michael Jacksons Kids Biologically His
Do Cells Lyse In Hypertonic Solutions
If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. In an isotonic environment, there is no net water movement, so there is no change in the size of the cell. The plasma membrane can only expand to the limit of the rigid cell wall, so the cell wont burst, or lyse.
Everyday Solutions All Around Us
If you’d like to see a hypertonic solution in your own home, add a slice of carrot to a cup of saltwater. You’ll find that it soon shrivels up as the water leaves the carrot cells for the hypertonic saltwater. Check out more examples of everyday solutions that you can find in your home, workplace or classroom. Or, if you’re interested in more medical terms, take a look at these common medical abbreviations.
You May Like: Paris Jackson Paternity
How Do You Lyse Cells For Dna Extraction
In lysis, the nucleus and the cell are broken open, thus releasing DNA. This process involves mechanical disruption and uses enzymes and detergents like Proteinase K to dissolve the cellular proteins and free DNA. The other step, which is known as precipitation, separates the freed DNA from the cellular debris.
Hypertonic Solution: Definition And Role In Cell Biology
A hypertonic solution refers to a solution that has a greater concentration of solute than another solution. In the context of biology, when two aqueous solutions are separated by a cell membrane, if the concentration of solute is greater outside the cell than inside the membrane, the solution is called hypertonic.
Thus, hypertonicity refers to a relative difference between the concentration of solute inside a cell and the concentration of the solute outside the cell. When a cell is placed in a hypertonic solution, osmotic pressure will force water out of the cell to balance the concentration of solute across the membrane.
Since water tends to flow out of the cell, cells placed in a hypertonic solution will shrink. The process by which water moves out a cell in a hypertonic solution is called plasmolysis. Cells that lose too much water can be damaged, and organisms immersed in strongly hypertonic solutions can become dehydrated. The other kinds of solutions relevant to osmosis are called hypotonic and isotonic. Hypotonic solutions have a lower concentration of solute than inside the cell so water rushes in, while isotonic solutions have an equal concentration of solute inside and outside the cell, so there is no net diffusion of water.
Read Also: Ccl4 Molecular Shape
What Does Hypertonic Mean In Biology
isishypertonicisis
. Similarly one may ask, what does hypotonic mean in biology?
Ahypotonic solution is any solution that has a lower osmotic pressure than another solution. In the biological fields, this generally refers to a solution that has less solute and more water than another solution.
Subsequently, question is, what happens when a cell is placed in a hypertonic solution? If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water . So if you get thirsty at the beach drinking seawater makes you even more dehydrated.
Furthermore, what is the difference between hypertonic and hypotonic?
1. Hypotonic solutions have less solutes and more solvent while hypertonic solutions have more solutes and less solvent. 2. Hypotonic solutions cause the cell to swell because it promotes shifting of water into it while hypertonic solutions cause the cell to shrink because it pulls the water out of the cell.
What does isotonic mean in biology?
An isotonic solution refers to two solutions having the same osmotic pressure across a semipermeable membrane. This state allows for the free movement of water across the membrane without changing the concentration of solutes on either side.
A Cell In Hypertonic Solution
The plasma membrane that surrounds cells is a special permeable membrane that separates the contents of the cell from the environment. The plasma membrane is embedded with special membrane transport proteins that help transport solutes across. It also has special protein channels called aquaporins that allow water to flow freely across the membrane. The cell must use energy to actively move solutes into and out of the cell. Too many solutes and the cytosol will become a hypertonic solution compared to the environment. Cells without cell walls can burst in this condition.
Too few solutes in the environment will become the hypertonic solution. In this case, the opposite will happen, as water moves out of the cell. Water moves against the concentration gradient of solutes, moving from areas of low solute concentration to areas of high solute concentration. In another sense, water moves with the water concentration gradient, from areas of high water concentration to areas of low water concentration.
Also Check: Is Ap Human Geography Hard
Is Hyperosmotic The Same As Hypertonic
Tonicityhypertonicis hyperosmotic
. Also, what is the difference between Hyperosmotic and Hypoosmotic?
Hyperosmotic: When one solution has a higher osmotic concentration than another. Hypoosmotic: When one solution has a lower osmotic concentration than another.
what is a Hyperosmotic solution? Hyperosmotic Definition. Hyperosmotic can refer to solutions that have increased osmotic pressure, or a greater difference between solutes and solutions between a membrane. In other instances, hyperosmotic refers to a solution that has more solutes, or components of a solution, than a similar solution.
Also to know is, can a Hyperosmotic solution be hypotonic?
Hypotonic: When cell has higher than solution water flows into cell causing it to swell. A hyperosmotic solution can be hypertonic, isotonic, or hypotonic depending on relative in cell and solution.
What is the difference between hypertonic and hypotonic?
Hypertonic refers to a greater concentration. In biology, a hypertonic solution is one with a higher concentration of solutes outside the cell than inside the cell. Hypotonic refers to a lesser concentration. In biology, a hypotonic solution has a lower concentration of solutes outside the cell than inside the cell.
What Happens When You Give Hypotonic Solution
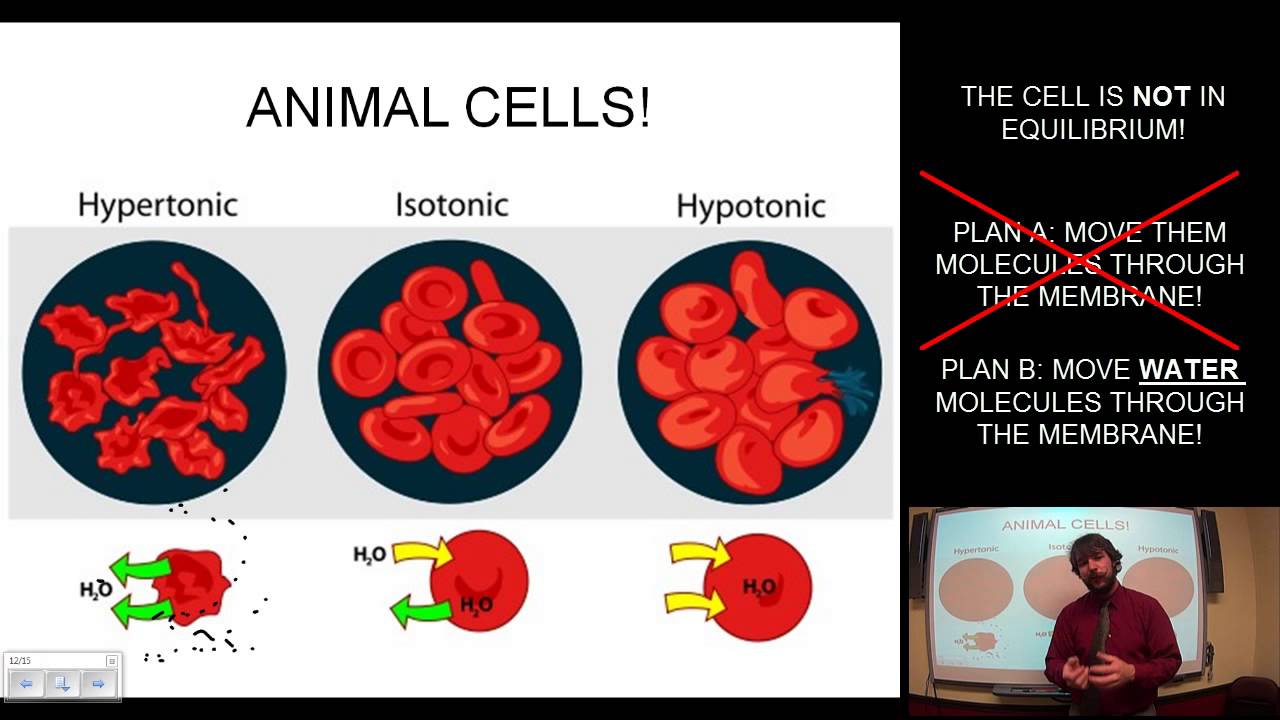
When a hypotonic solution is administered, it puts more water in the serum than is found inside cells. As a result, water moves into the cells, causing them to swell. This may cause the cells to swell and burst, exposing the veins basement membrane and potentially leading to phlebitis and infiltration.
Read Also: Ccl4 Lewis Structure
Difference Between Hypotonic And Hypertonic
July 9, 2012 Posted by Dr.Samanthi
The key difference between hypotonic and hypertonic is that hypotonic solution has a low solute concentration than the cell while hypertonic solution has a high solute concentration than the cell.
Osmosis is the process of moving water molecules from high water potential to low water potential through a semi-permeable membrane. However, this semi-permeable membrane only allows solvent particles to move across it and does not allow solute particles to move through the membrane. Tonicity is a measure of the osmotic pressure gradient and there are three states of it. These are hypertonic, isotonic and hypotonic. Among the three solutions, hypotonic solution is the solution which has a low solute concentration while hypertonic solution is the solution which has a high solute concentration. The solvent concentration gradient across the two solutions is the driving force for this process. The net movement of the solvent from hypotonic solvent to hypertonic solvent takes place due to the unequal osmotic pressure.
The Plasma Membrane And Cytosol
If the outside environment of a cell is water-based, and the inside of the cell is also mostly water, something has to make sure the cell stays intact in this environment. What would happen if a cell dissolved in water, like sugar does? Obviously, the cell could not survive in such an environment. So something must protect the cell and allow it to survive in its water-based environment. All cells have a barrier around them that separates them from the environment and from other cells. This barrier is called the plasma membrane, or cell membrane.
Also Check: Molecular Geometry Ccl4
Examples Of Hypertonic In A Sentence
hypertonic refinery29.comhypertonic Detroit Free Press
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘hypertonic.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
What Is Isotonic In Biology
The concept of isotonic can be used in different fields. In the field of chemistry, those solutions that have the same osmotic pressure when they are at the same temperature classifies as iso.
In hematology, solutions that have the same salt concentration as red blood cells are said to be an isotonic solution has. Therefore, they have the same osmotic pressure as blood and do not cause red blood cells to deform.
Isotonic
Applying this term to muscle contraction, a contraction is said to be isotonic when the muscle tension remains constant.
It should be remembered that a solution is a mixture that obtains when a solid dissolves in a liquid. The osmotic pressure, meanwhile, is the pressure exerted by the solvent particles on the semi-permeable membrane that establishes a separation with the more concentrated element. The temperature also is the physical quantity indicating the degree of heat of the environment or body.
Returning to the idea of isotonic, two or more solutions are iso when, at the same level of heat, the solvent particles of each exert the same pressure on the semipermeable membranes.
Heres the examples of isotonic. Lets look in detail.
Recommended Reading: Prince Jackson Real Father
Extracellular Fluid In Hypertonic Dehydration
Healthy blood cells have the same amount of water as the fluid around them. But if you sweat a lot or lose more water than sodium in other ways, your extracellular fluid is now hypertonic and you are dehydrated. Osmosis occurs between the fluid and the red blood cells, which depletes your blood cells and prevents them from carrying oxygen. Hypertonic dehydration can be mild to severe .
Uses Of Hypertonic Solutions
Manipulating the tonicity of a solution has practical applications. For example, reverse osmosis may be used to purify solutions and desalinate seawater.
Hypertonic solutions help to preserve food. For example, packing food in salt or pickling it in a hypertonic solution of sugar or salt creates a hypertonic environment that either kills microbes or at least limits their ability to reproduce.
Hypertonic solutions also dehydrate food and other substances, as water leaves cells or passes through a membrane to try to establish equilibrium.
Recommended Reading: Are Michael Jacksons Kids His Biological Kids
Why Students Get Confused
The terms “hypertonic” and “hypotonic” often confuse students because they neglect to account for the frame of reference. For example, if you place a cell in a salt solution, the salt solution is more hypertonic than the cell plasma. But, if you view the situation from the inside of the cell, you could consider the plasma to be hypotonic with respect to the saltwater.
Also, sometimes there are multiple types of solutes to consider. If you have a semipermeable membrane with 2 moles of Na+ ions and 2 moles of Cl- ions on one side and 2 moles of K+ ions and 2 moles of Cl- ions on the other side, determining tonicity can be confusing. Each side of the partition is isotonic with respect to the other if you consider there are 4 moles of ions on each side. However, the side with sodium ions is hypertonic with respect to that type of ions . The side with the potassium ions is hypertonic with respect to potassium . How do you think the ions will move across the membrane? Will there be any movement?
What you would expect to happen is that sodium and potassium ions would cross the membrane until equilibrium is reached, with both sides of the partition containing 1 mole of sodium ions, 1 mole of potassium ions, and 2 moles of chlorine ions. Got it?
Key Takeaways: Hypertonic Definition
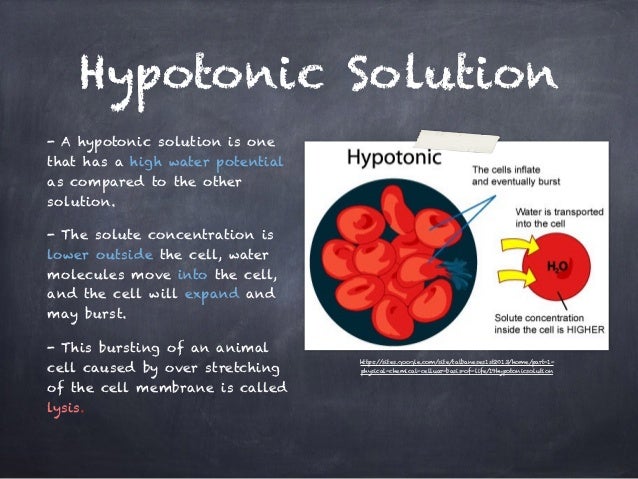
- A hypertonic solution is one which has a higher solute concentration than another solution.
- An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water.
- When two solutions are in contact, solute or solvent moves until the solutions reach equilibrium and become isotonic with respect to each other.
Recommended Reading: Simplifying Radicals Imaginary Numbers Worksheet Kuta Software
Diffusion Osmosis And Tonicity
In order to fully understand hypertonicity, we need to step back and look at the basic behavior of fluids in certain environments.
For any sample of fluid, the molecules in that fluid are subject to random motion. Over time, this random motion will compound, and the molecules in the fluid will change from being close together to being evenly spread apart. Diffusion is the name for this tendency for molecules in a fluid to move from regions of high concentration to regions of low concentration. Diffusion is a result of the random motion of molecules and does not require any net input of energy to occur. For example, if I put a drop of ink in a cup of water, over time, the ink will naturally spread out to be evenly mixed with the water.
Animals and plant cells have a selectively permeable membrane around them that lets some chemicals pass and keeps other things out . The term osmosis refers to the diffusion of water across a selectively permeable membrane. Osmosis is extremely important for living organisms as it regulates the amount of water inside and outside of cells. The presence of a semipermeable membrane affects what kinds of molecules it can diffuse across.
Given that there are three possible directions for the net water flow during osmosis, out, in, and neutral, we have three possible kinds of tonicity, labeled: hypertonic, hypotonic, and isotonic.
What Is Difference Between Hypotonic And Hypertonic Solution
A cell placed in a hypotonic solution will swell due to the movement of water into the cell. Alternatively, if a cell is placed in a hypertonic solution, the cell will shrink due to the movement of water outside the cell through osmosis. The difference between a hypotonic solution and a hypertonic solution is tabulated below:
| Hypotonic solution | Hypertonic solution |
| The solution outside the cell has a higher soluble concentration than inside the cell. Thus, the water molecules move outside the cell from inside. | The solution outside the cell has a less concentrated solution than inside the cell. Thus, the water molecules move inside the cell from outside. |
| A solution whose concentration is less than the cell sap or inside of a cell. | A solution whose concentration is more than the cell sap or that inside a cell. |
| A plant cell becomes turgid when putting in a hypotonic solution. | A plant cell undergoes plasmolysis in a hypertonic solution. |
Also Check: Does Kamala Harris Have Children