Why Do Most Atoms Form Chemical Bonds
Most elements contain atoms that form chemical bonds. This is because those atoms become more stable when they are bonded together. Neighbouring atoms are attracted to each other by electrical forces which make them stick together. Atoms that are strongly attractive rarely spend much time on their own other atoms will usually bond to them quite quickly. The arrangement of electrons around a central atom is what determines the strength in which it seeks out other atoms to bond with.
O2 Lewis Structure Molecular Geometry And Hybridization
O2 is a chemical element within the 16th group of the periodic table, called chalcogens. Besides being one of the simplest elements existing on this planet, oxygen is of great importance on the Earth.
Due to this reason, it is essential to study its Lewis structure. The existence of a strong shared covalent double bond between the two oxygen molecules within a single O2 molecule makes it crucial to study the Lewis structure even more.
Molecular Orbital Diagram Of O2
The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top.
The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule.
It takes a lot of energy to pair up the electrons within the same orbital. So, the diagram having no unpaired electrons is at higher energy.
It means it is at a much higher excited state than the other.
Also Check: Evaluating Functions Algebra 2 Worksheet
Bond Angles And Three
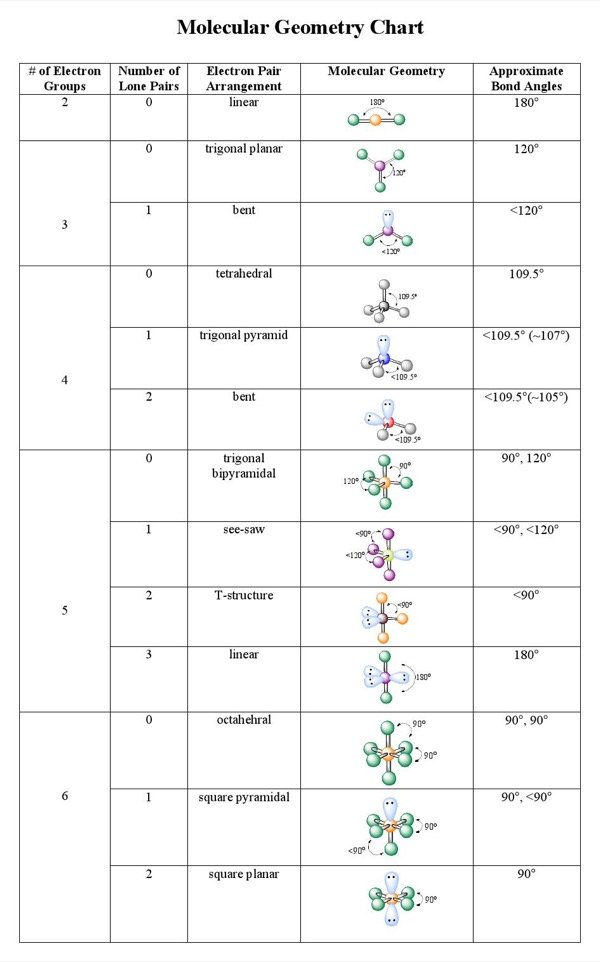
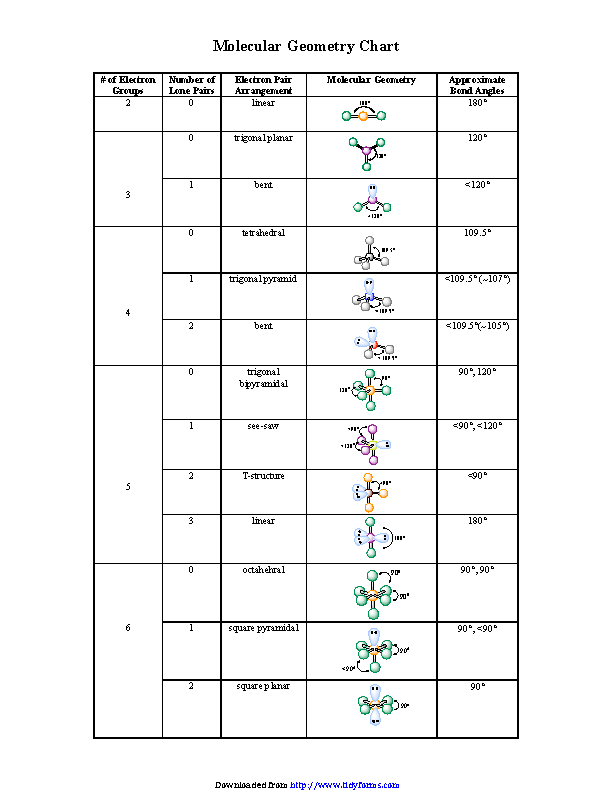
Students often make the mistake of thinking in two dimensions when determining bond angles. Electrons will position themselves to be as far away from each other as possible, but keep in mind that they will do this on a three-dimensional plane. A molecular geometry chart with bond angles will help to clarify these structures.
Working with three-dimensional models is a great way to get used to thinking about geometry in a three-dimensional plane. There are also apps and software you can use to create virtual models and get used to these concepts.
Vsepr And More Than Six Electron Pairs
It is considerably less easy to draw a distinction between apparently reasonable seven coordinate geometries. There are several possibilities, including the pentagonal bipyramid and the capped octahedron.
The pentagonal bipyramid and the monocapped octahedron.
Iodine heptafluoride, IF7, is a good example of a pentagonal bipyramidal geometry. The molecule XeF6 is an interesting case. As with IF7, application of VSEPR rules suggests seven electron pairs. These are made up from six bonding pairs and one lone pair. In fact, the structure of XeF6 is based upon a distorted octahedron, probably towards a monocapped octahedron. It is difficult to settle the geometry of the lowest energy configurtion because the geometry of XeF6 changes rapidly with time, that is, it is fluxional. The effect of this fluxional process is to average all the fluorine positions.
With higher coordination numbers the situation is more complex. For instance, the idealized geometry for eight electron pairs is a square antiprism but the energy of other coordination geometries may be very similar or more stable in particular cases.
The idealized coordination geometry for eight electron pairs.
A VSEPR tutorial on the WWW
Recommended Reading: Glencoe Geometry Chapter 8 Test Form 2b Answer Key
What Is Molecular Geometry
Molecular geometry describes the three-dimensional structure of a molecule. Chemists are able to predict the arrangement of atoms and chemical bonds using the valence-shell electron-pair repulsion theory or VSEPR. This theory revolves around the idea that electrons repel each other and therefore will bond accordingly.
Types of configurations and angles
There are three main types of configurations: linear, trigonal, and tetrahedral. Below is a table demonstrating the relationship between the number of bonding partners and these configurations.
| Configuration |
| 109.5 |
Determining molecular geometry and bond angles
To determine the molecular geometry of a structure we need to know two things. Firstly, we must know how many total attachments there are. additionally, we need to know how many of these attachments are bonds and lone pairs. Notice in the table below how if there are no lone pairs, the molecular geometry and electron geometry will be the same.
In the table below, you will see the coordination between the number and type of attachments in relation to the bond angles. For the most part, this information will have to be memorized.
| Attachments | |
| 3 | bent |
| 4 | trigonal pyramidal |
| 4 | bent |
Silicon Tetrachloride Lewis Dot Structure Molecular Geometry Polar Or Non
Home > Chemistry Article> SiCl4 lewis structure and its molecular geometry
Silicon tetrachloride is an inorganic compound that appears as a colorless liquid with a pungent odor having the chemical formula SiCl4. It can react with water violently and forms white solid silicon dioxide and HCl gas. It is soluble in water.
In this article, we will discuss Silicon tetrachloride lewis structure, molecular geometry, hybridization, polar or nonpolar, etc.
Silicon tetrachloride is corrosive to tissue and metal. It is used to produce high-quality silica for commercial purposes. High purity of silicon tetrachloride used in the manufacture of optical fibers.
Properties of Silicon tetrachloride
You May Like: Holt Geometry Chapter 7 Test Form A
How Two Pairs Of Valence Electrons Are Shared Between O2
When an atom is scarce of valence electrons, it readily either accepts or donates electrons to achieve a stable condition.
The lower the number of valence electrons needed, the tendency to donate the valence electrons increases.
As the oxygen atom requires only two valence electrons, it readily shares them with another oxygen atom which is also in need of two valence electrons.
The bond which is formed between a shared pair of electrons is a covalent bond.
As there is a stable balance between the attractive and repulsive forces due to the sharing of the electrons, the covalent bond formation is tough to break.
Moreover, as there is a formation of two covalent bonds between four valence electrons of the O2 atom, it becomes highly stable and is not easy to bond with the O2 atom without the presence of any catalyst.
What Is The Molecular Geometry Of Sicl4
The molecular geometry of SiCl4 is tetrahedral as all four outer atoms are pushed away in all directions from the central atoms because of repelling force occurs in electron pairs around the central atom.
Tetrahedral means having four faces.
The four Si-Cl bonds in SiCl4 geometry take the place of the regular tetrahedron corner, hence, its shape really looks like tetrahedral.
According to the VSEPR theory, when a central atom is attached to four bonded atoms then an electron pair around the central atom repel each other as a result all corners atoms spread out as much as they can and takes the place where the repulsion is minimum and stability is much better.
As you see in the above structure, the electron pairs repulsion occur, and the surrounding atoms push atom as much as they can to maximize distance and take the place where repulsion force between these are minimum.
So, all these spreaded atoms hold the place of regular tetrahedron corner, hence we can say, the molecular geometry of SiCl4 is tetrahedral.
If you cant visualize the molecular geometry of SiCl4, then theoretically we can use an AXN method and VSEPR chart to determines its shape.
Thats how the AXN notation follows as shown in the above picture.
Now we have to find the molecular geometry of SiCl4 by using this method.
AXN notation for SiCl4 molecule:
So, the AXN notation for the SiCl4 molecule becomes AX4N0 or AX4.
Hence, the molecular shape or geometry for SiCl4 is tetrahedral.
Recommended Reading: Segment Addition Postulate Practice
An Example Of Molecular Geometry
We will use H2O as an example.
H2O is a common polar molecule. The central atom in this case is the oxygen atom which has six valence electrons. Hydrogen, in this case, gives a total of two electrons, which makes the total overall amount of electrons eight. In this example, there are two lone electron pairs, and four electron groups, as well as two single bond pairs. Therefore, the molecular geometry in this example is bent.
Significance Of Lewis Structure
Lewis structure is a diagrammatic representation of showing the bond formation between the atoms of molecules.
Furthermore, this structure also helps with determining the lone electrons existing within the molecule and how they will be acting in a bond formation.
This diagram shows bonds with the help of lines and lone pairs of electrons as dots.
The Lewis structure helps with understanding how electrons are distributed within a compound along with its molecular geometry.
Besides this, the lewis structure helps with determining the hybridization of the molecule.
You May Like: Is Michael Jackson Biological Father
Molecular Geometry Chart: Definition Examples And Study Guides
How much do you know about molecular geometry definition and the shapes of molecules in chemistry? Join us as we define this subject, go over some examples, and list the different structures you will find in an electron and molecular geometry chart. We have also included some study guides to help you go further.
H2o Molecular Geometry Lewis Structure Shape And Bond Angles
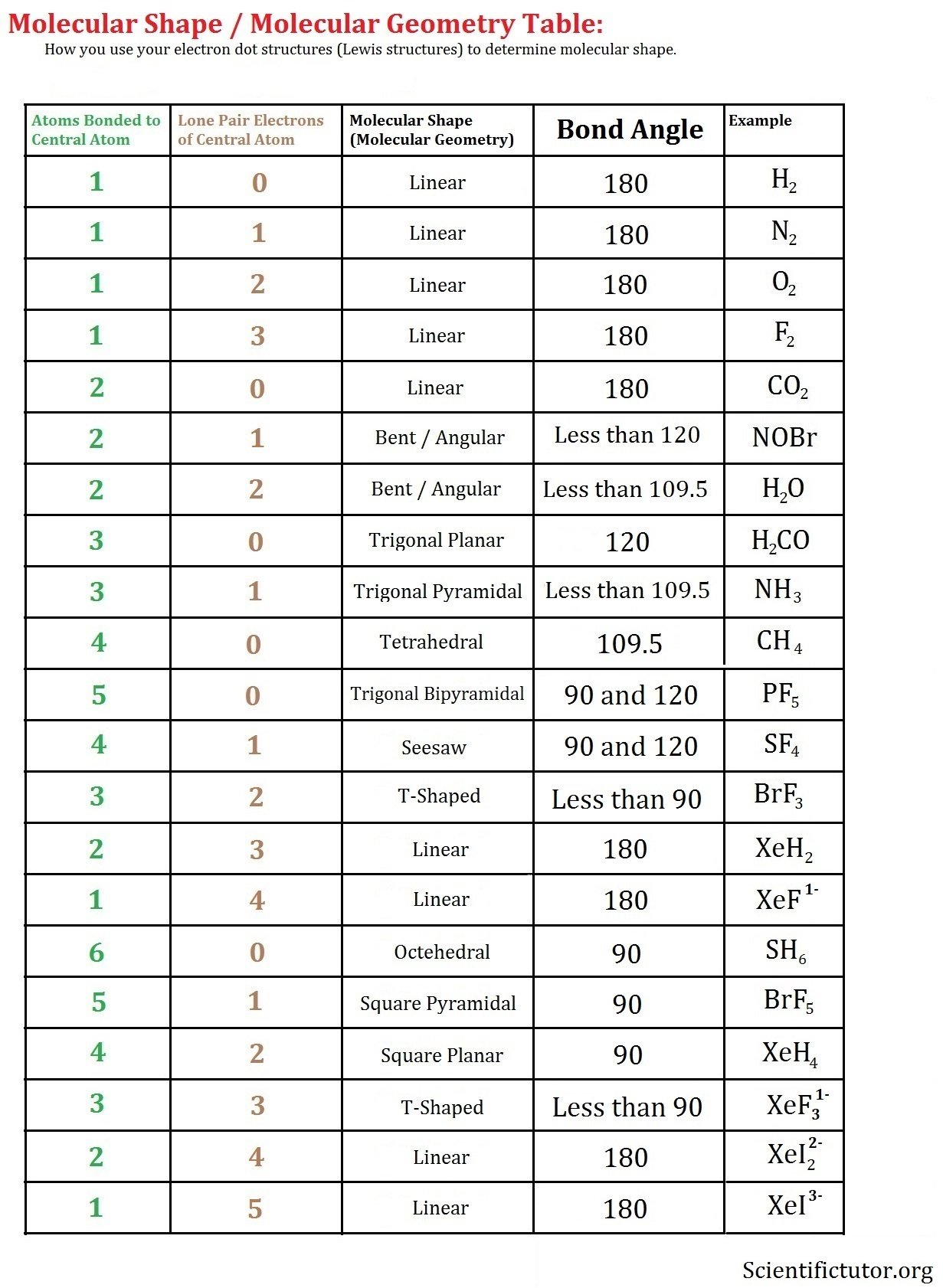
We have previously discussed the Lewis structures of CO2, O3, SO2, SO3 and more. Today we are going to learn about the Lewis structure of H2O molecule along with its molecular geometry and shape.
Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. While we always knew chemistry is everywhere, we definitely didnt know that even water has chemical formulas back in our childhood days. Water has a chemical formula of H2O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide.
| Name of molecule | |
| No of Valence Electrons in the molecule | 8 |
| Bent |
& nbsp
In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry and Bond angles. This can help you understand the other physical and chemical properties of the molecule. But before looking at its Lewis Structure, we will first go through the total number of valence electrons for this molecule as these electrons are the ones that participate in bond formation.
Contents
Don’t Miss: Geometry Seeing Doing Understanding Answer Key Pdf
How Many Lone Pair And Bonded Pair Electrons A Lewis Structure Of Sicl4 Contains
The central atom is attached to the chlorine atoms with four single bonds.
One bonded pair contains two electrons, hence, = 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride.
Also, lone pair electrons are also called unshared electrons, silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it.
Hence, = 12 lone pairs.
= 24 lone pair electrons are present in the lewis structure of SiCl4.
What Are The Valence Electrons
The valence electrons are present in the outermost shell of an atom which participates in a bond formation.
This bond formation further leads to the formation of a molecule and eventually a compound.
The valence electrons either get accepted or donated to form a strong bond, usually which is covalent in most of the cases.
Don’t Miss: Parallax Errors
Why Is The Molecular Geometry Of Sicl4 Is Same As Its Electron Geometry
The molecular geometry of SiCl4 is tetrahedral and its electron geometry is also tetrahedral because as per VSEPR theory, molecular shape considers only bond pairs or atoms while electron geometry considers bonded atoms as well as lone pairs present on the central atom.
According to the lewis structure of SiCl4, the central atom doesnt contain any lone pair on it.
Hence, only bonded atoms are used to determine the geometry of SiCl4.
Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4
How Many Valence Electrons/lone Pair Of Electrons Are In O2
The analysis of the number of valence electrons present in one oxygen molecule is done with the help of the electronic configuration.
The atomic number of one oxygen atom is eight where its electronic configuration shows a deficiency of 2 electrons in the 2p shell.
It concludes to the fact that oxygen needs two electrons to achieve a stable condition.
The octet rule says that the elements bond in such a manner that they tend to achieve a maximum of eight electrons in their outermost shell or the valence shell.
As we studied above that one oxygen atom has a deficiency of two valence electrons, it readily accepts two electrons.
So, a single oxygen molecule has six electrons in its octet. If we look for O2, then the number will be O2: 6+6 = 12. In total, an O2 molecule needs four valence electrons to complete its octet and achieve a stable condition.
Recommended Reading: Holt Geometry Workbook Answer Key
The Influence Of Thermal Excitation
Since the motions of the atoms in a molecule are determined by quantum mechanics, “motion” must be defined in a quantum mechanical way. The overall quantum mechanical motions translation and rotation hardly change the geometry of the molecule. In addition to translation and rotation, a third type of motion is molecular vibration, which corresponds to internal motions of the atoms such as bond stretching and bond angle variation. The molecular vibrations are harmonic , and the atoms oscillate about their equilibrium positions, even at the absolute zero of temperature. At absolute zero all atoms are in their vibrational ground state and show zero point quantum mechanical motion, so that the wavefunction of a single vibrational mode is not a sharp peak, but an exponential of finite width . At higher temperatures the vibrational modes may be thermally excited , but they oscillate still around the recognizable geometry of the molecule.
To get a feeling for the probability that the vibration of molecule may be thermally excited,we inspect the Boltzmann factorβ â¡ exp, where ÎE is the excitation energy of the vibrational mode, k the Boltzmann constant and T the absolute temperature. At 298 K , typical values for the Boltzmann factor β are:
- β = 0.0890 for ÎE = 0500 cmâ1
- β = 0.0080 for ÎE = 1000 cmâ1
- β = 0.0007 for ÎE = 1500 cmâ1.
Molecules With More Than One Atom
The molecular geometry chart still applies if you have a molecule with more than one atom. The structure will be more complex and will probably combine different geometric shapes.
If you have a complex molecule, break it down into smaller section and look at each atom individually. Determine how the electrons will connect to this atom in function of the type of bond they form and of the number of electron groups.
You can use molecular geometry rules to determine the shape and structure of each atom and its electrons. You can then apply the Valence-shell electron-pair repulsion theory to determine how these different small structures will connect to each other to form a more complex molecule.
Recommended Reading: Algebra Nation Section 9 Practice Book Answers
Why There Is A Strong Covalent Bond In O2
A single covalent bond is usually made up of a sigma bond which is the strongest covalent bond, due to head-on overlapping between the shared pair of valence electrons.
After , comes the Pi bond, which is weaker than the bond, as they occur because of lateral overlapping between the shared pair of electrons.
As O2 has one and one bond, because of which valence electrons in it undergoes both head-on and lateral overlapping.
Due to this reason, O2 is a stable molecule. The below-mentioned diagram is showing sigma and pi overlapping within the oxygen molecule.
Here, an oxygen molecule is formed due to the occurrence of overlapping between the two partially-filled p-orbits within each of the oxygen atoms .
Silicon Tetrachloride Polarity: Is Sicl4 Polar Or Nonpolar
A polar molecule is asymmetrical contains lone pair and has some dipole moment whereas non-polar molecules are highly symmetrical contain no unshared electrons and have net dipole moment zero.
So, Is SiCl4 polar or nonpolar? SiCl4 is a nonpolar molecule in nature as its shape is highly symmetrical, also its central atom doesnt contain any lone pair, hence distortion of shape doesnt happen.
If you see the molecular geometry of SiCl4, all four chlorine atoms are equally spaced around the silicon atom in a tetrahedron corner. Hence, the dipole moment generated along the bond can be easily canceled out, leaving this molecule nonpolar in nature with net dipole moment zero.
Dipole moment generated along with the bond due to the separation of charge induced on atoms, this charge is induced because the electronegativity of chlorine is 3.16 and for silicon, it is 1.90. The difference of the electronegativity between these atoms high, this makes a Si-Cl polar covalent bond in nature.
Although the bonds within a molecule are polar in nature but the structure of SiCl4 is highly symmetrical, this causes a uniform charge distribution in the whole molecule.
Hence, canceling dipole in SiCl4 becomes a lot easy leaving this molecule nonpolar in nature.
Recommended Reading: Holt Algebra 1 Chapter 7 Cumulative Test Answers
Table Of Three To Six Electron Domains
1 For the General Molecular Formula,”A” refers to central atom, “X” refers to atoms attached tocentral atom, and “E” refers to unbonded electron pairs. Forexample, AX2E2 is shown as the formula for water, which has two bonded hydrogen atoms and twolone electron pairs .
2 For compounds containing 5 pairs ofelectrons , all lone pairs are placed inthe trigonal planar electron region, never above or below the trigonal planarregion. This arrangement maximizes the separation of lone electronpairs to their neighbors. Seethe Trigonal bipyramidal webpage for diagrams.
3 For Hybrid Orbitals, you canalways know the VSEPR electron arrangement . Molecular Geometry is based on the arrangementof the bonded atoms, according to the General Molecular Formulacolumn.
4 For molecules that contain the same attached atoms,a symmetrical molecule is not polar. An assymetrical molecule is polar if the individual bondsare polar.