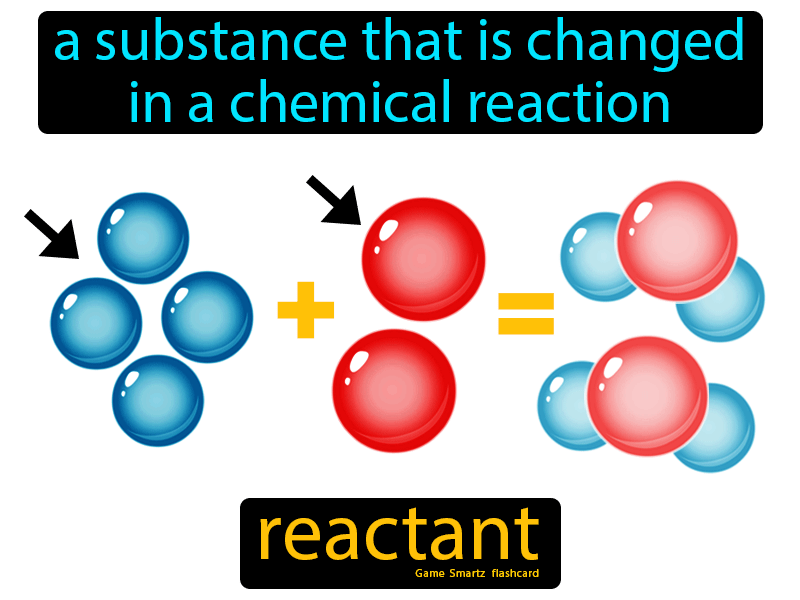
What Is Limiting Reagents
The reactant that is entirely used up in a reaction is called as limiting reagent.
Limiting reagents are the substances that are completely consumed in the completion of a chemical reaction. They are also referred to as limiting agents or limiting reactants. According to the stoichiometry of chemical reactions, a fixed amount of reactants is required for the completion of the reaction. Let us consider the following reaction of formation of ammonia:
3H2 + N2 2NH3
In the reaction given above, 3 moles of Hydrogen gas are required to react with 1 mole of nitrogen gas to form 2 moles of ammonia. But what if, during the reaction, only 2 moles of hydrogen gas are available along with 1 mole of nitrogen.
In that case, the entire quantity of nitrogen cannot be used . Hence, the hydrogen gas is limiting the reaction and is therefore called the limiting reagent for this reaction.
Overview Of Excess Reactant In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The excess reactant is the reactant in a chemical reaction with a greater amount than necessary to react completely with the limiting reactant. It is the reactant that remain after a chemical reaction has reached equilibrium.
Identifying Reactants And Products In Chemical Equations
Look at the reaction arrow to identify the reactants and products in a chemical equation. In a reaction that only proceeds in the forward direction, the arrow points from left to right. The reactants are to the left of the arrow, while the products are to the right of the arrow. If any chemical species are listed on both sides of the equation , they are neither reactants nor products.
In the following reaction, A and B are reactants and C is the product:
A + B C
However, there doesnt need to be more than one reactant. In this reaction, A is the reactant, while B and C are products:
A B + C
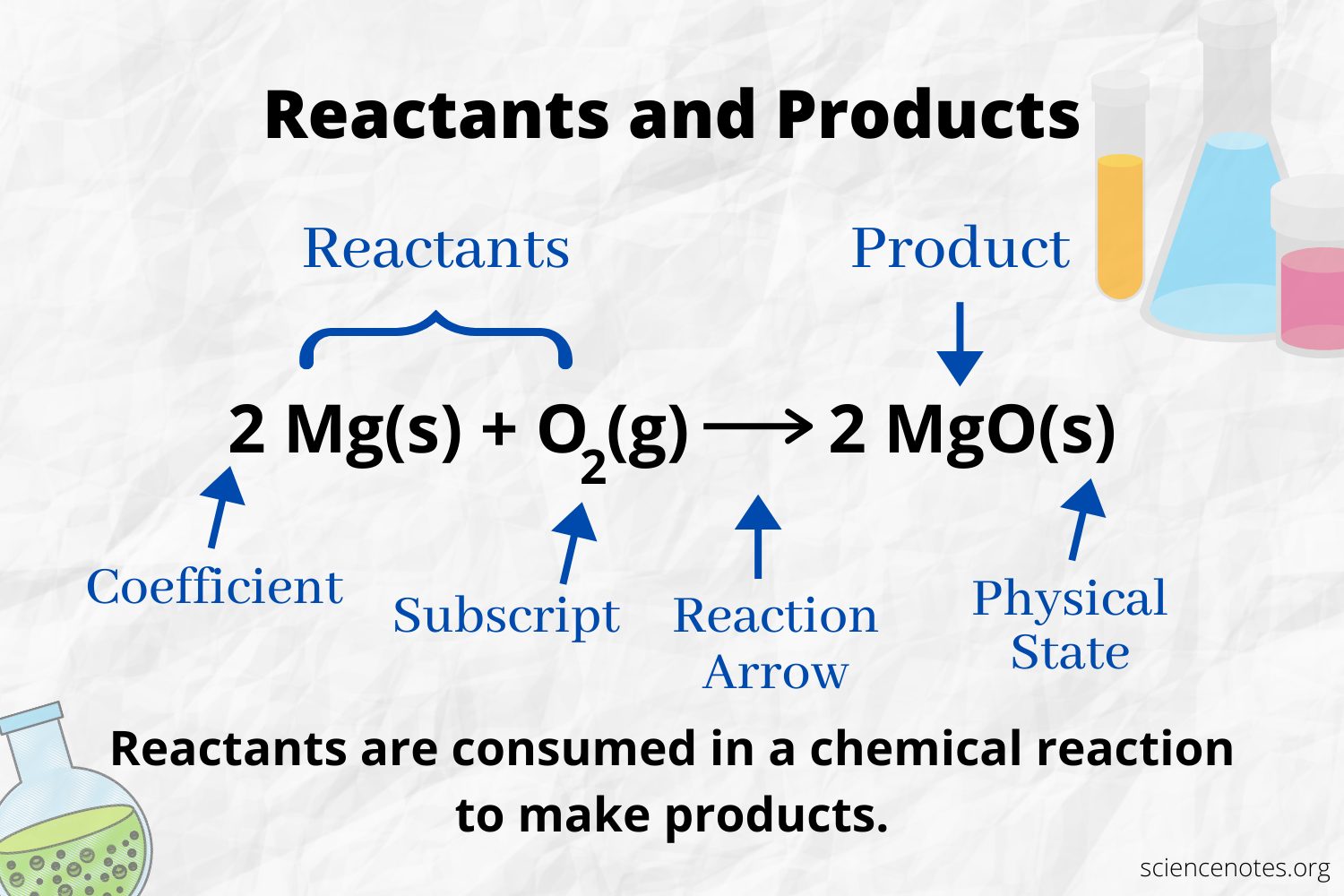
The number and type of atoms is the same for the products and the reactants in a balanced chemical equation. For example, the number of hydrogen and oxygen atoms is the same for the reactants and product .
2 H2 + O2 2 H2O
The number of each type of atom is its coefficient multiplied by its subscript . So, there are 4 atoms of hydrogen on the reactant side and 2 atoms of oxygen . There are 4 atoms of hydrogen on the product side and 2 atoms of oxygen . The state of matter is stated following each chemical formula.
Many reactions proceed in both directions to reach an equilibrium state. Here again, the reaction arrow identifies the reactants and products, but the arrow points both ways! In this type of reaction, the chemical species on each side of the reaction are both reactants and products.
An example is the Haber process, which forms ammonia from nitrogen and oxygen:
Also Check: How To Find Ksp Chemistry
Concentration Affect The Rate Of Reaction
Reaction involving twoparticles
According to the collision theory, the reaction is the same whether the collision between two different particles or collisions between the same particles. Particles first collide in any reaction, whether both particles are in solution or one in solution or other one is solid. On the higher concentration, higher will be the collision.
Reaction involving oneparticle
The orientation of the collision of the molecules isirrelevant. Suppose that at any one time 2 in a million particles have enoughenergy to equal or exceed the activation energy. If you have 300 millionparticles then 300 of them would react. By doubling the concentration, doublewill be the rate of reaction.
How To Find Limiting Reagent
The determination of the limiting reactant is typically just a piece of a larger puzzle. In most limiting reactant stoichiometry problems, the real goal is to determine how much product could be formed from a particular reactant mixture. The limiting reactant or reagent can be determined by two methods.
In order to calculate the mass of the product first, write the balanced equation and find out which reagent is in excess. Using the limiting reagent calculate the mass of the product.
The following points should be considered while attempting to identify the limiting reagent:
- When there are only two reactants, write the balanced chemical equation and check the amount of reactant B required to react with reactant A. When the amount of reactant B is greater, the reactant A is the limiting reagent.
- The reactant which is in a lesser amount than is required by stoichiometry is the limiting reactant.
- In an alternate method of finding the limiting agent, the amount of product formed by each reactant is calculated.
- The limiting reactant is the reactant from which the minimum amount of product is formed.
- Also, if we calculate the amount of one reactant needed to react with another reactant, then the reactant which is in shortage would be the required limiting reactant.
Recommended Reading: Geometry Segment And Angle Addition Worksheet
Main Difference Reactant Vs Reagent
A chemical reaction involves the reaction between two or more compounds to make one or more new compounds. In other words, a chemical reaction is the change of reactants in order to form products. These reactants can be in solid phase, liquid phase or gaseous phase. The term reagent is used to describe a type of reactants. Reagents are added to a reaction mixture for the progression of the reaction. However, unlike reactants, reagents are not necessarily changed into some other compound. Thus, the main difference between reactant and reagent is that reactants are consumed in chemical reactions whereas reagents are not necessarily consumed during the progression of a reaction.
Examples Of Reactant In A Sentence
reactantQuartzQuartzreactant Ars TechnicareactantSFChronicle.comreactant Popular MechanicsreactantsArs Technicareactants Ars Technicareactants Ars Technica
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘reactant.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Read Also: How To Convert In Chemistry
What Would You Do Without A Computer
What would you do without a computer?
Over the last few decades, computers have proven to be extremely useful tools for organizing and processing information. You put data into the computer and your output can be a detailed diagram of how that molecule looks in three dimensions. The computer and its programs transform input data into a useful final product.
Chemical Reactions Take Different Forms
The major types of chemical reaction are:
- Synthesis or Direct Combination Reaction – Reactants form a more complex product.
- – A reactant breaks into two or more smaller products.
- Single Displacement or Replacement Reaction – Also called a substitution reaction, this occurs when the ion from one reactant changes place with another.
- Double Displacement or Replacement Reaction – Also called a metathesis reaction, this occurs when both cations and anions of the reactants trade places to form products.
While some reactions involve a change in the state of matter , a phase change is not necessarily an indicator of a reaction. For example, melting ice into water is not a chemical reaction because the reactant is chemically identical to the product.
Reaction Example: The chemical reaction H 2 + ½ O 2 H 2O describes the formation of water from its elements.
You May Like: Who Are Paris Jackson’s Biological Parents
What Is A Reactant In Chemistry Definition And Examples
In chemistry, a reactant is a starting material in a chemical reaction that is consumed to form products. The activation energy required to initiate a chemical reaction breaks the bonds between reactant atoms. The reactant undergoes a chemical change, forming new bonds that result in products. The term reactant first came into use around 1900 to 1920.
What Is The Benefit Of Having A Limiting Reagent
In a chemical reaction, the task of limiting reagent or reactant is significant because it can help the chemist predict the maximum quantity of reactant is consumed, since it restricts the reaction, only the necessary moles of products can be produced instead of the hypothetical yield where the perfect quantity is used.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: Houghton Mifflin Geometry Worksheet Answers
What Is Concentration Of Reactants
The increase in the concentration of reactants, the rate ofreaction increases. Ions and molecules interact to form a new compound onincreasing the concentration. The decrease in the concentration of reactants,fewer molecules and ions are present and the rate of reaction decreases.
In the reaction of gases, if we increase the pressure therewill be an increase in concentration and the rate of reaction also increases.
Example
Degenerated reaction of calcium carbonate obtained by thepollutant sulfur dioxide. The amount of sulfur dioxide in the air depends onthe rate of reaction. SO2 is an acidic oxide, it combines with watervapor to produce H2SO3. The reaction is as follows:
SO2 + H2O = H2SO3
Then calcium carbonate reacts with sulfurous acid
CaCO3 + H2SO3 = CaSO3 + CO2 + H2O
The concentration of Sulfur dioxide is high in a pollutedair. In the polluted air the calcium carbonate more degenerates as compared to lesspolluted air.
On the other hand, Phosphorus burns at a great rate in a pureoxygen than in air.
Unit
Concentration is usually measured in moles per liter.
Different Values Of Reaction Order
As discussed earlier, the value of the order of reaction may be in the form of an integer, zero, or a fraction. A graph detailing the reaction rates for different reaction orders can be found below.
Chemical reactions can be classified into the following types based on the dependence of the rate on the concentration.
Also Check: Algebra 2 Eoc Fsa Practice Test
Excess Reactant Limiting Reactant And Theoretical Yield
Because atoms, molecules, and ions react with each other according to molar ratios, you’ll also encounter stoichiometry problems that ask you to identify the limiting reactant or any reactant that is present in excess. Once you know how many moles of each reactant you have, you compare this ratio to the ratio required to complete the reaction. The limiting reactant would be used up before the other reactant, while the excess reactant would be the one leftover after the reaction proceeded.
Since the limiting reactant defines exactly how much of each reactant actually participates in a reaction, stoichiometry is used to determine theoretical yield. This is how much product can be formed if the reaction uses all of the limiting reactant and proceeds to completion. The value is determined using the molar ratio between the amount of limiting reactant and product.
What Are The Basics Of Chemical Reactions
- A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products. Substances are either chemical elements or compounds.
- A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. The properties of the products are different from those of the reactants.
- Chemical reactions differ from physical changes, which include changes of state, such as ice melting to water and water evaporating to vapor. If a physical change occurs, the physical properties of a substance will change, but its chemical identity will remain the same.
Also Check: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Is A Reactant And Product In Science
Reactants and Products in Chemical Reactions. Ina chemical reaction, substances calledreactants are changed into other substances called products. You can’t change oneelement into another in a chemical reaction that happens innuclear reactions.
Also know, what is the definition of reactant and product?
From Reactants to ProductsReactants are substances that start a chemicalreaction. Products are substances that are produced in thereaction.
Likewise, what are examples of reactants and products?From Reactants to ProductsWhen a candle burns, the reactants are fuel and oxygen . The productsare carbon dioxide gas and water vapor.
In this manner, what are products in science?
In chemistry, a product is a substance that isformed as the result of a chemical reaction. In a reaction,starting materials called reactants interact with eachother.
What is an example of reactant?
Under the proper conditions, such as temperature, time,or pressure, the chemical bonds of the reactants are broken,and the atoms form new bonds that give different combinations. Thesubstances that result from this recombination of atoms are calledthe products of the reaction. Example– NH3 + HCL NH4Cl.
You May Like Also
How Are Chemical Reactions Classified
Chemists classify chemical reactions in a number of ways: by type of product, by types of reactants, by reaction outcome, and by reaction mechanism. Often a given reaction can be placed in two or even three categories, including gas-forming and precipitation reactions. Many reactions produce a gas such as carbon dioxide, hydrogen sulfide, ammonia, or sulfur dioxide. Cake batter rising is caused by a gas-forming reaction between an acid and baking soda . Classification by types of reactants include acid-base reactions and oxidation-reduction reactions, which involve the transfer of one or more electrons from a reducing agent to an oxidizing agent. Examples of classification by reaction outcome include decomposition, polymerization, substitution, and elimination and addition reactions. Chain reactions and are examples of classification by reaction mechanism, which provides details on how atoms are shuffled and reassembled in the formation of products.
chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituentatoms of the reactants to create different substances as products.
Read Also: Homework 2 Segment Addition Postulate Answer Key
Difference Between Molecularity And Order Of Reaction
The molecularity of a reaction refers to the number of atoms, molecules, or ions which must undergo a collision with each other in a short time interval for the chemical reaction to proceed. The key differences between molecularity and reaction order are tabulated below.
| Molecularity | |
| It is always a whole number | It can be a whole number or a fraction. |
| It can be determined from the balanced chemical equation | It must be determined experimentally |
| Is only applicable in simple reactions | The reaction order is applicable in all chemical reactions |
It can be noted that when the order of reaction is a fraction, the reaction is generally a chain reaction or follows some other complex mechanism. An example of a chemical reaction with a fractional reaction order is the pyrolysis of acetaldehyde. This reaction has an order of 1.5.
To learn more about the order of reaction and other concepts related to chemical kinetics, register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Definition Of Reaction Rate
The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. The speed of a chemical reaction may be defined as the change in concentration of a substance divided by the time interval during which this change is observed:
For a reaction of the form \, the rate can be expressed in terms of the change in concentration of any of its components
\}\]
\}\]
\}\]
in which \ is the difference between the concentration of \ over the time interval \:
\ = _2 _1 \label\]
Notice the minus signs in the first two examples above. The concentration of a reactant always decreases with time, so \ and \ are both negative. Since negative rates do not make much sense, rates expressed in terms of a reactant concentration are always preceded by a minus sign to make the rate come out positive.
Consider now a reaction in which the coefficients are different:
It is clear that \ decreases three times as rapidly as \, so in order to avoid ambiguity when expressing the rate in terms of different components, it is customary to divide each change in concentration by the appropriate coefficient:
\} = -\dfrac = \dfrac \label\]
Example \: Oxidation of Ammonia
For the oxidation of ammonia
it was found that the rate of formation of N2 was 0.27 mol L1 s1.
Solution
3 × = 0.81 mol L1 s1.
2 × = 0.54 mol L1 s1.
Also Check: Lesson 8.1 Practice B Geometry Answers
How To Identify The Excess Reactant
The excess reactant may be found using the balanced chemical equation for a reaction, which gives the mole ratio between reactants.
For example, if the balanced equation for a reaction is:
2 AgI + Na2S Ag2S + 2 NaI
You can see from the balanced equation there is a 2:1 mole ratio between silver iodide and sodium sulfide. If you start a reaction with 1 mole of each substance, then silver iodide is the limiting reactant and sodium sulfide is the excess reactant. If you are given the mass of reactants, first convert them to moles and then compare their values to the mole ratio to identify the limiting and excess reactant. Note, if there are more than two reactants, one will be a limiting reactant and the others will be excess reactants.