Ario Atom Resonance Induction & Orbital
When were looking at compounds in Organic Chemistry and were looking at acidity or basicity, I really want you to remember four;general principles.
These are related to the letters A, R, I, and O:
- A;stands for;Atom
- I;is;Induction
- O;is;Orbital
This is the general order in which I want you to think of in terms of looking at the acidity of a compound. A is more important generally than R, R is more important generally than I, and O is normally the last on the list.
Now, this becomes more clear when we look at examples. Lets look at a few examples.
The More Stable A Pair Of Electrons Is The Less Basic It Will Be The Less Stable A Lone Pair Of Electrons Is The More Basic It Will Be
Notice the role that each of these anions plays in these reactions: it is accepting a proton from water, so in other words it is acting as a base.
Therefore, our whole discussion of the; stability of anions,; for lack of a better term, goes by another name youre familiar with: basicity.;
In other words:
- the more stable a lone pair of electrons is, the less basic it will be.
- the less stable a lone pair of electrons is, the more basic it will be.
Quantitative Measurement Of Acid And Base Strength
- You should know that the dissociation of a Bronsted-Lowry acid in water produces the conjugate base of the Bronsted acid, along with the hydronium ion. The equilibrium constant K for this dissociation measures the extent of hydronium ion formation and thus, effectively , how strong the Bronsted acid is. In this case strength refers to the relative tendency of the acid to protonate water.
- The acid dissociation constant of the Bronsted-Lowry acid is the quantity which is actually used to measure the strength of the acid quantitatively . This is simply the product of the hydronium ion concentration and the concentration of the conjugate base of the acid , divided by the concentration of the Bronsted acid itself. Notice that the concentration of water, which is also on the left hand side of the equation, is omitted from the expression, since this is effectively constant in dilute aqueous solution. Thus the acid dissociation constant is the equilibrium constant K multiplied by the constant concentration of water in water.
- Frequently, the pKa, which is the negative common log of the acid dissociation constant is used as a more conveniently-sized number to measure the acidity. Since this is the negative log, a large positive pKa means a large negative exponent of the acid dissociation constant, Ka. this corresponds to a weak acid . Conversely, a negative value of the pKa corresponds to a strong acid.
Also Check: Lesson 9.4 Practice B Geometry Answers
Amine Extraction In The Laboratory
Extraction is often employed in organic chemistry to purify compounds. Liquid-liquid extractions take advantage of the difference in solubility of a substance in two immiscible liquids . The two immiscible liquids used in an extraction process are the solvent in which the solids are dissolved, and the extracting solvent. The two immiscible liquids are then easily separated using a separatory funnel. For amines one can take advantage of their basicity by forming the protonated salt , which is soluble in water. The salt will extract into the aqueous phase leaving behind neutral compounds in the non-aqueous phase. The aqueous layer is then treated with a base to regenerate the amine and NaCl. A second extraction-separation is then done to isolate the amine in the non-aqueous layer and leave behind NaCl in the aqueous layer.
Basicity Trend #: Basicity Increases With Increasing Negative Charge On Nitrogen
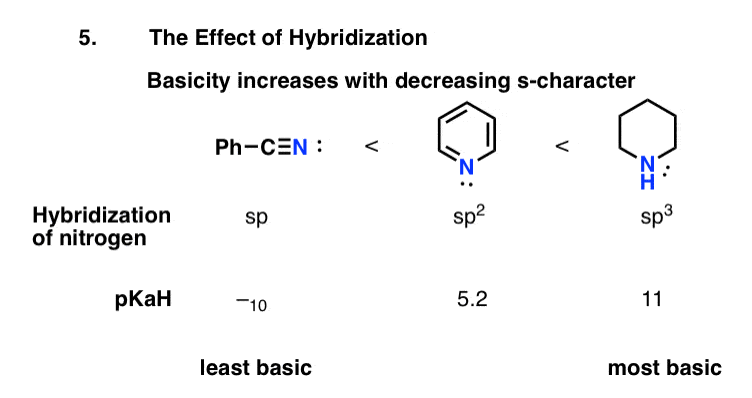
This is possibly the simplest factor to evaluate. If basicity can roughly be translated as electron-pair instability, and instability increases with charge density, then basicity should increase with increased negative charge.
A simpler way to put it: the;conjugate base of an amine will always be a stronger base than the amine itself.
Compare ammonia, with its conjugate base, the amide anion NH2. The amide anion is stronger base by far . It can be used to deprotonate terminal alkynes , for example, whereas ammonia will not.
Continuing this trend, the conjugate base of the amide ion, the amide dianion NH should be an even stronger base, but it seems to be prohibitively difficult to make. ;;.;
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Brnsted Acidity And Basicity
In 1923, chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently developed definitions of acids and bases based on the compounds’ abilities to either donate or accept protons ions). In this theory, acids are defined as proton donors; whereas bases are defined as proton acceptors. A compound that acts as both a Brønsted-Lowry acid and base together is called amphoteric.
Basicity Of Substituted Arylamines
The addition of substituents onto the aromatic ring can can make arylamines more or less basic.;Substituents which are electron-withdrawing decrease the electron density in the aromatic ring and on the amine making the arylamine less basic.;In particular, the nitro group of para-nitroaniline allows for an additional resonance form to be drawn, which further stabilizes the lone pair electrons from the nitrogen, making the substituted arylamine less basic than aniline.;This effect is analogous to the one discussed for the acidity of substituted phenols inSection 17.2.
Substituents which are electron-donating increase the electron density in the aromatic ring and on the amine making the arylamine more basic.;In the case of para-methoxyaniline, the lone pair on the methoxy group donates electron density to the aromatic system, and a resonance contributor can be drawn in which a negative charge is placed on the carbon adjacent to the nitrogen, which makes the substituted arylamine more basic than aniline.
Increased Basicity of para-Methoxyaniline due to Electron-Donation
Exercise s\
1) Using the knowledge of the electron donating or withdrawing effects of subsituents gained in Section 16.6, rank the following compound in order of decreasing basicity.a) p-Nitroaniline, methyl p-aminobenzoate, p-chloroanilineb) p-Bromoaniline, p-Aminobenzonitrile, p-ethylaniline
You May Like: Geometry Segment Addition Postulate Worksheet
What Is The Priority Order Of Factors Like Resonance Hybridization Solvation Etc In Determining The Relative Acidity Or Basicity Of Compounds In A List
WARNING! Long answer! Here are my thoughts.
Explanation:
Anything that decreases the #”X-H”# bond strength in the acid or stabilizes the conjugate base will increase the acidity and decrease the basicity of the conjugate base.
I would identify the factors in the order 1. #bb”R”#
There are six common strong acids. In order of increasing acidity, they are:
#”HNO”_3 < “H”_2″SO”_4 < “HCl” < “HBr” < “HI” < “HClO”_4#
Any strong acid is stronger than most organic acids.
Some factors to consider are:
I put resonance stabilization of the anion first in the list.
The more resonance contributors a conjugate base has, the stronger the acid will be.
That partly explains why sulfuric acid is stronger than nitric acid.
It also explains why acetic acid is a stronger acid thanethanol (
Basicity Of Nitrogen Groups
In this section we consider the relative basicity of amines. When evaluating the basicity of a nitrogen-containing organic functional group, the central question we need to ask ourselves is: how reactive is the lone pair on the nitrogen? In other words, how much does that lone pair want to break away from the nitrogen nucleus and form a new bond with a hydrogen. The lone pair electrons makes the nitrogen in amines electron dense, which is represents by a red color in the electrostatic potential map present below left. Amine are basic and easily react with the hydrogen of acids which are electron poor as seen below.
Amines are one of the only neutral functional groups which are considered basis which is a consequence of the presence of the lone pair electrons on the nitrogen. During an acid/base reaction the lone pair electrons attack an acidic hydrogen to form a N-H bond. This gives the nitrogen in the resulting ammonium salt four single bonds and a positive charge.
Amines react with water to establish an equilibrium where a proton is transferred to the amine to produce an ammonium salt and the hydroxide ion, as shown in the following general equation:
The equilibrium constant for this reaction is the base ionization constant , also called the base dissociation constant:
\} \label\]
pKb = -log Kb
Consider the reactions for a conjugate acid-base pair, RNH3+ RNH2:
\}}\]
\}}\]
Adding these two chemical equations together yields the equation for the autoionization for water:
Recommended Reading: What Is Copulation In Biology
What Is Inductive Effect
When an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms , the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect.
An illustration describing the inductive effect that arises in a chloroethane molecule due to the more electronegative chlorine atom is provided above.
Also Read
Note That This Section Is Quite Different Form The One In Our Text For The Purposes Of This Course Please Neglect The Text Discussion And Consider The Present Discussion As Replacing Itwe Are Again Considering Ways In Which The Anion Produced By Deprotonating A Bronsted
- The pKa of acetic acid is 4.76, while that of chloroacetic acid is 2.86, i.e., the latter is almost 100 times more acidic than the former.
- The conjugate base in each case is a carboxylate anion, so electronegativity effects and resonance effects should be equal in each case. What makes the chloroacetate anion relatively more stable than the acetate ion? This is the inductive effect.
- We recall that a C-Cl bond is substantially polar in the sense of carbon being partially positively charged and chlorine partially negatively charged, because of the electronegativity difference betwee these two atoms. The dipole in this bond is oriented with its positive end closer to the two sites of negative charge. There is therefore a stabilizing electrostatic attraction between the positive end of the C-Cl dipole with the negative charge of the anion. This is larger than the destabilizing interaction of the negative charge with the chlorine end of the diple, because the distance between the latter and the negatively charged oxygens is greater. Thus, there is a net electrostatic attraction and stabilization in the anion because of the existence of the dipole and the orientation of the positive end toward the negatively charge oxygens.
- If the dipole were oriented with the negative end toward the anion site, there would be a destabilization, and a decrease in acidity.
Also Check: Algebra Road Trip Project Answer Key
Inductive Effects In Nitrogen Basicity
Alkyl groups donate electrons to the more electronegative nitrogen. The inductive effect makes the electron density on the alkylamine’s nitrogen greater than the nitrogen of ammonia. The small amount of extra negative charge built up on the nitrogen atom makes the lone pair even more attractive towards hydrogen ions. Correspondingly, primary, secondary, and tertiary alkyl amines are more basic than ammonia.
| Compound |
| 9.81 |
Acidity And Basicity Of Amines
After completing this section, you should be able to
Key Terms
Make certain that you can define, and use in context, the key term below.
- amide
Study Notes
The lone pair of electrons on the nitrogen atom of amines makes these compounds not only basic, but also good nucleophiles. Indeed, we have seen in past chapters that amines react with electrophiles in several polar reactions .
The ammonium ions of most simple aliphatic amines have a pKa of about 10 or 11. However, these simple amines are all more basic than ammonia. Why? Remember that, relative to hydrogen, alkyl groups are electron releasing, and that the presence of an electronreleasing group stabilizes ions carrying a positive charge. Thus, the free energy difference between an alkylamine and an alkylammonium ion is less than the free energy difference between ammonia and an ammonium ion; consequently, an alkylamine is more easily protonated than ammonia, and therefore the former has a higher pKa than the latter.
Don’t Miss: Geometry Segment Addition Postulate Worksheet
Basicity Trend #3 Inductive Effects Decrease Basicity
You may recall that electron withdrawing atoms or functional groups tend to increase acidity, by slurping away electron density from the conjugate base. Trifluoroethanol ;for example, is far more acidic than ethanol itself .; Lower charge density = more stability = lower basicity.;
Hence, wed expect that electron withdrawing groups on amines should likewise decrease their basicity. And they do! Witness morpholine compared to piperidine , or 2-chloropyridine versus pyridine .
How To Determine The Stronger Acid
In Organic Chemistry, acids and bases is really quite qualitative. Were often looking at whether a compound is even acidic or basic at all. Or if we compare two compounds, which one is more acidic or more basic or which part of an organic compound is acidic or basic? Watch the video below to find out!
Read Also: Eoc Fsa Warm Ups Algebra 1 Answers
Inductive Effect Vs Electromeric Effect
A tabular column highlighting the key differences between the electromeric and the inductive effects can be found below.
| Inductive Effect | |
| The inductive effect is permanent | The electromeric effect is a temporary effect |
| It doesnt require any attacking reagent | An electrophilic attacking reagent is required for this effect to arise. |
Thus, it can be understood that the +I and -I effects play a vital role in the stability as well as the acidity or basicity of molecules.
Basicity Is Just Another Word For Stability Of A Lone Pair Of Electrons
Last time I started writing about acid-base reactions. We looked at this list of stabilities of anions going across the topmost row of the periodic table.
Fluoride ion is the most stable in this series because its the most electronegative; carbon is the least stable because its the least electronegative.
Because of this, we were able to say that H-F was the most acidic, because it had the most stable conjugate base.
And H-CH3 was the least acidic, because it had the least stable conjugate base.
Lets look at the flip side of this reaction. Instead of starting with HF, H2O, H3N, and CH4 and asking how likely they are to donate a proton to a common base , imagine we start with the anions and have them take a proton away from ;a common acid .
Recommended Reading: Exponential Growth And Decay Worksheet Algebra 1
A Case Where Bond Strength Effects Predominate Over Anion Stability Effects
- The pKa’s of H-F,H-Cl,H-Br, and H-I are respectively 3.5,-7,-8,and -9. That is, H-F is the weakest acid and H-I the strongest (recall that a negative pKa corresponds to a strong acid.
- Based upon anion stability, the opposite trend should be observed, since fluoride ion should be the most stable of the halide anions
- However, the H-F bond is extremely strong and hard to break, so the bond strength effect is acid weakening and is predominant here. The bond dissociation energies of the H-X bonds are, respectively, 136 ,103 ,88 , and 71 . So, the bond strength differences, being large, are greater than the differences in anion stability, causing the former effect to predominate. The effect is largest for fluorine, so that there is relatively little difference in the acidity of the other halogen acids. Thus the two effects are almost, but not quite in balance for these latter three H-X’s.
- Similar considerations apply for comparisons in any case involving atoms in the same Group of the Periodic Table. But, rarely are bond strength effects dominant over anion stability effects in any other comparative situation.
Comparing The Basicity Of Alkylamines To Amides
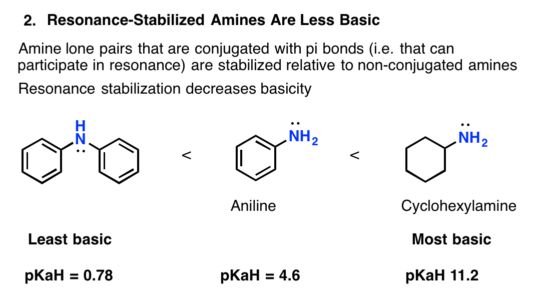
The nitrogen atom is strongly basic when it is in an amine, but not significantly basic when it is part of an amide group. While the electron lone pair of an amine nitrogen is localized in one place, the lone pair on an amide nitrogen is delocalized by resonance. The electron density in the form of a lone pair is stabilized by resonance delocalization, even though there is not a negative charge involved. Heres another way to think about it: the lone pair on an amide nitrogen is not as available for bonding with a proton these two electrons are too stable being part of the delocalized pi-bonding system. The electrostatic potential map shows the effect of resonance on the basicity of an amide. The map shows that the electron density, shown in red, is almost completely shifted towards the oxygen. This greatly decreases the basicity of the lone pair electrons on the nitrogen in an amide.
Don’t Miss: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Inductive Effect On Acidity And Basicity
Using the inductive effect, we can predict the acidity and basicity of compounds. As a generalisation, it may be said that the electron-withdrawing groups increase the acidity of a compound and electron-donating group decrease the acidity of a compound.
This is because, if we take the conjugate base of the acid, that is, RCOO-, if R is electron-withdrawing, then the conjugate base is stabilised via delocalisation of the formed negative charge.
If R had been electron-donating, then the conjugate base would be destabilised because of inter-electronic repulsions.
Thus, it can be said that, +I groups decrease acidity and I groups increase acidity of compounds.
For Example, formic acid is more acidic than acetic acid due to the +I inductive effect of the methyl group attached to the carboxylic acid group.
Note: If Ka of acid is high, it is a strong acid, but if PKa of acid is high, it is said to be a weak acid Same logic applies to bases.
Consider, the acidity of mono-, di- and trichloroacetic acid.
It can be said that the presence of three Cl atoms make oxygen highly electron deficient and thereby, polarising the O-H bond the most. Therefore, the acidity order for the above compounds would be, III > II > I.