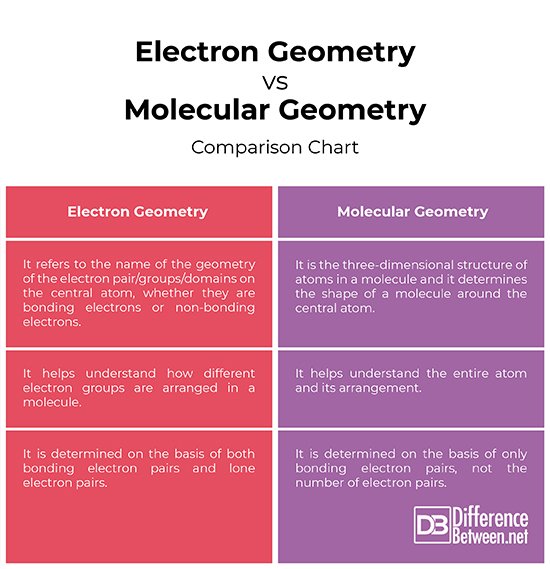
What Is Geometry Of A Molecule
The geometry of a molecule is the structure of the molecule, including both lone electron pairs and bond electron pairs of the central atom. Therefore, this term is different from the shape of a molecule because the shape of a molecule is determined using only the bond electron pair, excluding the lone electron pairs.
Figure 02: Geometry of a Water Molecule
There are different methods of determining the geometry of a molecule, such as various spectroscopic methods, diffraction methods, etc.
Difference Between Electron And Molecular Geometry
1. The geometry in which the shape of a molecule is predicted by considering both bond electron pairs and lone electron pairs is called electron geometry. The geometry in which the shape of a molecule is predicted by considering only bond electron pairs is called molecular geometry.
2. In electron geometry, lone electron pairs are considered. In molecular geometry, lone electron pairs are not considered.
3. In electron geometry,number of total electron pairs should be calculated. In molecular geometry, number of bonding electron pairs should be considered.
What Are The Differences In Shapes Between Molecular Geometry And Electronic Geometry
In my chemistry course we are told that molecular geometry considers only atoms as part of the shape, while electronic geometry considers atoms and electrons as part of the shape. However, I can’t find any difference between the two shapes for methane online. All I can find is this:
It confuses me that there can even be another shape for this because isn’t the molecular geometry already take into consideration the fact that each molecular wants to be as far away from one another as possible? What is the difference in shape between electronic and molecular geometry?
- 3$\begingroup$The term electron geometry is something which you will find in general chemistry books only . Don’t take it too literally. The is that if electron pairs are being considered, you call it electron geometry.$\endgroup$;M. FarooqMar 10 ’19 at 1:44
- $\begingroup$Electronic geometry and shapes might be used loosely as many terms. The fact that you post the Q let me think that the meaning is somehow clear to you, and you just make sure to not misunderstand something. At a more general lexical level, electronic geometry would better refer to the electron density size and shape around atoms/molecules, and this never show up in straight edges and vertices. Something like in the pic here researchgate.net/figure/ anyway ans below are correct$\endgroup$
Read Also: Segment Addition Postulate And Midpoint Worksheet Answer Key
What Is Electron Geometry
Electron geometry is the shape of a molecule predicted by considering both bond electron pairs and lone electron pairs. The VSEPR theory states that electron pairs located around a certain atom repel each other. These electron pairs can be either bonding electrons or non-bonding electrons.
The electron geometry gives the spatial arrangement of all the bonds and lone pairs of a molecule. The electron geometry can be obtained using VSEPR theory.
What Is A Vsepr Formula
The AXE method of electron counting is commonly used when applying the VSEPR theory. The electron pairs around a central atom are represented by a formula AXnEm, where A represents the central atom and always has an implied subscript one. Each E represents a lone pair of electrons on the central atom.
You May Like: Lesson 9.4 Practice B Geometry Answers
How Do You Write Electron Geometry
There are three basic steps to determining the molecular shape of a molecule:
Difference Between Molecular Geometry And Electron Geometry
| Molecular geometry | Electron geometry |
| Molecular Geometry is the arrangement of atoms in a molecule, normally relative to a single central atom. | Electron Geometry is the arrangement of electron pairs around a central atom. |
| It excludes lone pairs in deciding the shape of a molecule, although repulsion from lone pair is taken into account only in bond angles. | It considers the presence of both bond pair and lone pair of electrons in determining the shape. |
You May Like: Fsa Algebra 1 Eoc Review Functions And Modeling-answer Key
Is Electron Domain Geometry And Molecular Geometry
electronElectron domain geometrieselectronmolecular geometriesmolecule
Electron geometry teaches us about the arrangement of different electron groups. Molecular geometry, on the other hand, helps us understand the entire atom and its arrangement. It is the 3D arrangement of all the atoms in a particular molecule.
Also Know, why is so2 bent and not linear? CO2 is linear and the SO2 structure is bent, because CO2 has a negative oxygen on each side of the positive carbon they cancel each other out. In the SO2 structure the oxygen are not in lined with each other which means there a positive and negative end.
One may also ask, how do you find the electron domain and molecular geometry?
Count the total number of electron domains. Use the angular arrangement of the chemical bonds between the atoms to determine the molecular geometry. Keep in mind, multiple bonds count as one electron domain. In other words, a double bond is one domain, not two.
What is the molecular geometry of h2o?
Water or H2O has 8 electrons around the central oxygen atom. This means there are four electron pairs arranged in a tetrahedral shape. There are two bonding pairs and two lone pairs. The resulting shape is bent with an H-O-H angle of 104.5°.
Central Atoms With Five Bonds
Compounds of the type AX5 are formed by some of the elements in Group 15 of the periodic table; PCl5 and AsF5 are examples.
In what directions can five electron pairs arrange themselves in space so as to minimize their mutual repulsions? In the cases of coordination numbers 2, 3, 4, and 6, we could imagine that the electron pairs distributed themselves as far apart as possible on the surface of a sphere; for the two higher numbers, the resulting shapes correspond to the regular polyhedron having the same number of sides. The problem with coordination number 5 is that there is no such thing as a regular polyhedron with five vertices.
Note: Regular Polyhedra
In 1758, the great mathematician Euler proved that there are only five regular convex polyhedra, known as the platonic solids: tetrahedron , octahedron , icosahedron , cube , and dodecahedron . Chemical examples of all are known; the first icosahedral molecule, \ was prepared in 1986.
Besides the five regular solids, there can be 15 semi-regular isogonal solids in which the faces have different shapes, but the vertex angles are all the same. These geometrical principles are quite important in modern structural chemistry.
Figure: trigonal bipyramid structure
In 5-coordinated molecules containing lone pairs, these non-bonding orbitals will preferentially reside in the equatorial plane. This will place them at 90° angles with respect to no more than two axially-oriented bonding orbitals.
Read Also: What Is Copulation In Biology
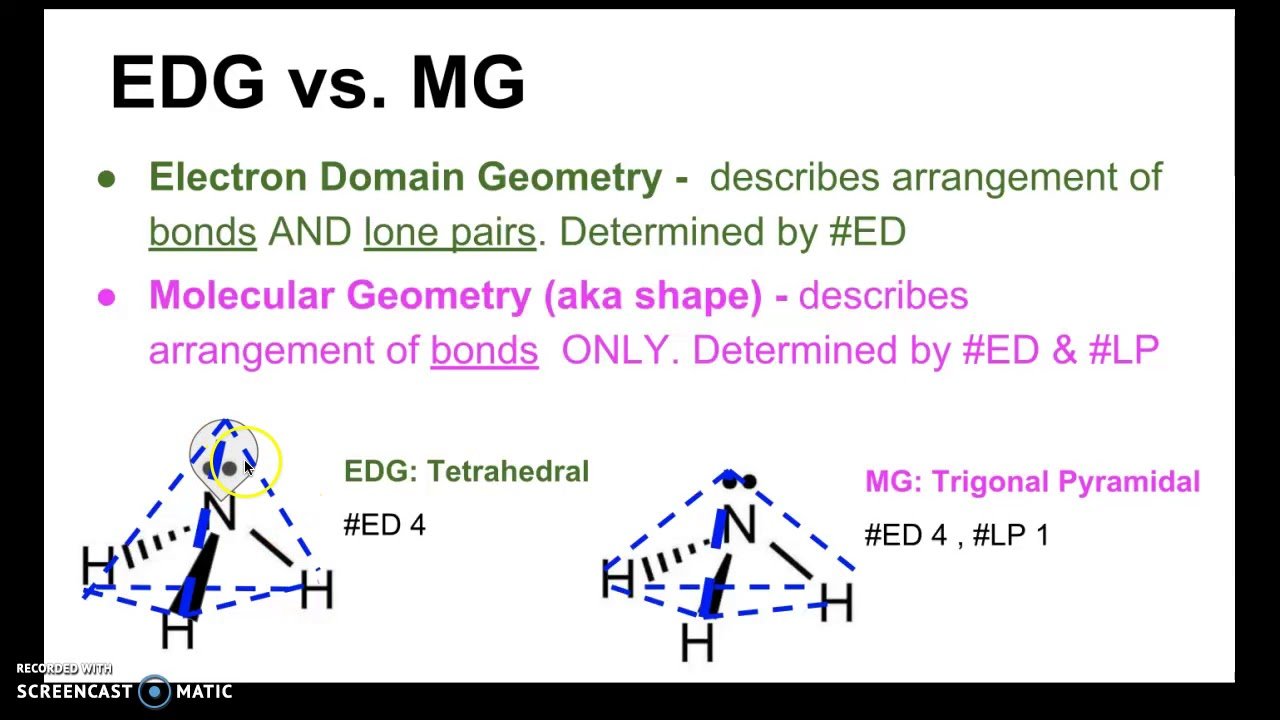
What Is The Difference Between Electronic And Molecular Geometries
See Below
Explanation:
Electron Geometry is the shape the electrons take around the central atom. These electrons can be in either a chemical bond or in lone pairs.- Linear
Molecular GeometryThis is the shape the actual connections between atoms take in a compound. The shape is dictated by the electron geometry, though. The electron geometry is the scaffold, and the connections between the atoms depend on this scaffold.
Ammonia has electron geometry that is tetrahedral . It has a lone pair, though, and if you put this lone pair on top, then the connections between N-H make the NH3 compound look like a camera on a tripod – this is called “Trigonal Pyramidal” – and this is its molecular geometry.
What Is The Relationship Between Bonding Bond Angle And Molecular Geometry
So, if a molecule has only two electron pairs, like that in CO2 , the two bond pairs repel each other and move the farthest from each other. Two objects can only be the farthest away from each other if they are in a linear structure, so the bond angle in such molecules is 180o and the molecule has a linear shape.
Recommended Reading: Draw The Lewis Structure For Ccl4
What Are The Electron And Molecular Geometry Of Clf3
According to VSEPR , ClF3 molecular geometryis T-shaped and its electron geometry is trigonal bipyramidal.;
There are two lone pairs present on the central atom of ClF3 molecule Which is adjusted on the axial plane to maximize the bond angle and minimize the bond repulsion. Two F are adjusted on an equatorial position separated by an angle of 180°. And one is adjusted on the axial plane in a see-saw way. It acquires a trigonal bipyramid structure but lone pairs are not a part of the structure. Hence the structure of ClF3 is a T-shaped structure.
As you see in the above structure, two lone pairs on central atom repelling each other, also, they tried to repel bonded pair electrons as well, as a result, both fluorine atoms in the equatorial position pushed far apart from each other giving the look of T-Shaped structure.
Theoretically, We need ClF3 lewiss structure to help to determine its shape. Because the lewis diagram helps us to identify how many lone pairs and bond pairs a molecule contains.;
Lets see step by step how to determine the molecular and electron geometry of ClF3.
1. Find the Number of lone pairs present on the central atom of the ClF3 lewis structure
First of all, we need to find how many lone pairs ClF3 central atom contains. As we see in the ClF3 lewis structure, Chlorine which is the central atom contains 2 lone pairs.
Or we can find lone pair in ClF3 by using the direct formula:
L.P = /2
where L.P. = Lone pair on the central atom
/2
H = N.A. + L.P.
Molecular Geometries And Dipole Moments
From models of SF4, BrF3, and XeF4, deduce whether differentatom arrangements, called geometrical isomers, are possible, and,if so, sketch them below.Indicate the preferred geometry foreach case and suggest a reason for your choice.Indicatewhich structures have dipole moments, and show their direction.MoleculeDipole MomentPreferred GeometrySF4BrF3XeF4
Don’t Miss: Geometry Segment Addition Postulate Worksheet
Is Co3 A Trigonal Planar
Answer: The shape of CO3^2- is trigonal planar. It has the carbon in the middle with three oxygens bonded to it. One C-O bond is a double bond, while the other two C-O bonds are single bonds. The oxygens with the single bonds have three lone pairs each and the oxygen with the double bond has two lone pairs.
This Problem Has Been Solved
VSEPR theory includes two typesof geometries: electron geometry and molecular geometry. What isthe difference between the two geometries?
| -Electron geometry describes the arrangement of the electrongroups in a molecule, whereas the molecular geometrydescribes the arrangement of the atoms. |
| -There is no difference between electron and moleculargeometries; they are simply different names for the samething. |
| Lone pairs are included in the electron geometry, but play norole in the molecular geometry. |
| -The electron geometry only considers lone pairs of electronsand the molecular geometry only considers the atoms. |
Read Also: Exponential Growth And Decay Worksheet Algebra 1
What Is The Difference Between Shape And Geometry Of A Molecule
The key difference between shape and geometry of a molecule is that shape of a molecule is the structure of the molecule excluding the lone pair on the central atom whereas the geometry of a molecule describes the arrangement of lone pair and bond pair electrons around the central atom of the molecule. Usually, the terms shape and geometry of a molecule are used interchangeably because both these structures are typically the same for most molecules if there are no lone electron pairs on the central atom of the molecule.
Below infographic summarizes the differences between shape and geometry of a molecule.
Clf3 Molecular Geometry Or Shape
You can also use the AXN method to determine the molecular geometry or electron geometry of ClF3.
- A represents the central atom.
- X represents the bonded atoms to the central atom.
- N represents the lone pairs on the central atom
With the help of the ClF3 Lewis dot structure, we know chlorine is the central atom that contains 2 lone pairs and is attached to 3 bonded atoms.
So, the ClF3 formula becomes AX3N2.
According to the AX3N2 formula, ClF3 molecular geometry is T-shaped and electron geometry is trigonal pyramidal.
| Bonded atoms |
| Trigonal bipyramidal |
VSEPR Chart
As ClF3 has 2 lone pair or 3 bond repulsion units and it formed; T-shaped or trigonal pyramidal geometry, Its FClF involving the axial atoms bond angle is 175º and FClF involving the one axial atom and one equatorial bond angle is around 90º.
You May Like: What Is The Molecular Geometry Of Ccl4
Example : Molecular Simulation
Using molecular shape simulator allows us to control whether bond angles and/or lone pairs are displayed by checking or unchecking the boxes under Options on the right. We can also use the Name checkboxes at bottom-left to display or hide the electron pair geometry and/or molecular structure .
Build the molecule HCN in the simulator based on the following Lewis structure:
\text\equiv \text
Molecular Geometry Vs Electron Pair Geometry
Electron geometry is about the arrangement of electron groups. Molecular geometry helps us understand the number of lone pairs of electrons.;In electron geometry, we determine the molecular shape. In molecular geometry, we only consider the bond electron pairs.;
Electron geometry and molecular geometry are similar when all electrons are bonded and dont have lone pairs. If lone pairs those that are not bonded to any other atom are located in a molecule, this relatively changes the molecular geometry, and not the electron geometry.;
References:
Read Also: What Is The Molecular Geometry Of Ccl4
Difference Between Electron Geometry And Molecular Geometry
Science is the investigation of issues of the society and it manages the numerous ways one sort of issue can be changed into different sorts. All molecules are made out of three major particles protons, electrons, and neutrons. At the point when at least two particles are firmly held together to shape an atom, there are compound connections between every molecule and its nearby neighbors. The state of a particle passes on an abundance of data and the initial step to understanding the science of an atom is to know its calculation.
The calculation of an atom decides the reactivity, extremity and natural movement of that particle. The VSEPR hypothesis can be utilized to decide the calculations of atoms.
Difference Between Electron Pair Geometry And Molecular Geometry
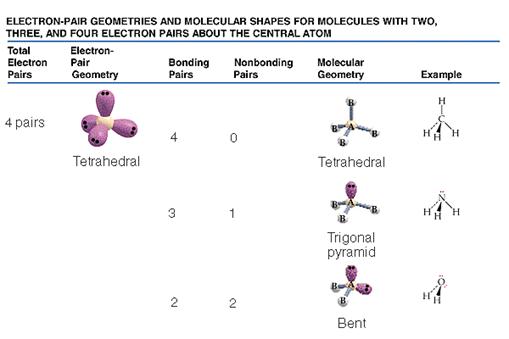
Electron Pair Geometry vs Molecular Geometry ;
The geometry of a molecule is important in determining its properties like color, magnetism, reactivity, polarity, etc. There are various methods of determining the geometry. There are many types of geometries. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, octahedral are some of the commonly seen geometries.
What is Molecular Geometry?
Molecular geometry is the three dimensional arrangement of atoms of a molecule in the space. Atoms are arranged in this way, to minimize the bond-bond repulsion, bond-lone pair repulsion and lone pair-lone pair repulsion. Molecules with the same number of atoms and electron lone pairs tend to accommodate the same geometry. Therefore, we can determine the geometry of a molecule by considering some rules. VSEPR theory is a model, which can be used to predict the molecular geometry of molecules, using the number of valence electron pairs. However, if the molecular geometry is determined by the VSEPR method, only the bonds should be taken into consideration, not the lone pairs. Experimentally the molecular geometry can be observed using various spectroscopic methods and diffraction methods.
What is Electron Pair Geometry?
Atoms in a molecule are bound together by electron pairs. These are called bonding pairs.
Some atoms in a molecule may also possess pairs of electron not involved in bonding. These are called lone pairs.
Lone pairs occupy more space than bonding pairs.
You May Like: What Is The Molecular Geometry Of Ccl4
S Used To Find The Shape Of The Molecule
To sum up there are four simple steps to apply the VSEPR theory.
Determination Of Molecular Geometry
The shape of a molecule is determined by the bonded atom, although this does not mean the shape itself is unaffected by the presence of lone pair.
Molecular geometry includes geometrical parameters such as bond lengths, bond angles, and torsional angles that help determine the position of atoms as well as a molecules general shape.
It influences a substances properties such as its reactivity, color, polarity, magnetism, biological activity, and phase of matter.
There are various techniques to determine molecular geometry such as Raman spectroscopy, infrared spectroscopy, and microwave spectroscopy.
Don’t Miss: What Is The Molecular Geometry Of Ccl4
How To Determine Electron Geometry
The following are the steps used in this determination.
Central atom of the molecule ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; =; ;;;C
Number of valence electrons of C ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;=;;;; 4
Number of electrons donated by hydrogen atoms ; =;; 4 x ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;;; =;; 4 x 1;; =; 4
Total number of electrons around C ; ; ; ; ; ; ; ; ; ; ; ; ; =;; 4 + 4 ;;;=;;; 8
Number of electron groups ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; =; 8 / 2;;;; =;;;; 4
Number of single bonds present ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;=; 4
Number of lone electron pairs ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;=; 4 4;;;; = 0
Therefore, the electron geometry ; ; ; ; ; ; ; ; ; ; ; ; ;; ; ; ; =;; tetrahedral
Figure 1: Electron Geometry of CH4
Electron Geometry of Ammonia
Central atom of the molecule ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; = N
Number of valence electrons of N ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; = 5
Number of electrons donated by hydrogen atoms ; ; = 3 x ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;; ;; = 3 x 1 = 3
Total number of electrons around N ; ; ; ; ; ; ; ; ; ; ; ; ; ;= 5 + 3 = 8
Number of electron groups ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; = 8 / 2 = 4
Number of single bonds present ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;= 3
Number of lone electron pairs ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;= 4 3 = 1
Therefore, the electron geometry ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; = tetrahedral
Figure 2: Electron Geometry of Ammonia
Electron Geometry of AlCl3
Central atom of the molecule ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;= Al
Number of valence electrons of Al ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; = 3