Amu And G/mol Relation
Do we have that $\pu = \pu$ ?
Because we have, for the mass of an atom of carbon 12, call it $m$, that
$$m = \pu$$
$$m = \pu = \pu$$
So finally we get that $\pu = \pu$ .
However, my chemistry teacher is telling me that those are two completely different things and that I am confused between the mass per atom and the mass per $6.022\cdot10^$ atoms. I cant understand how, and this is really bugging me, so help is very appreciated.
Note that this requires the mole to be a number , which may be where Im wrong.
You are correct, but to make it a little more clear you can include the assumed atom in the denominator of amu:
$$\beginm_^} &= \pu \\ \\m_^} &= \pu \\ \\\pu &= \pu \\ \\\pu &= \pu\end$$
In other words, the ratio of amu/atom is the same as the ratio of g/mol. The definitions of amu and moles were intentionally chosen to make that happen . This allows us to easily relate masses at the atomic scale to masses at the macroscopic scale.
To check this, look at the mass of an amu when converted to grams:
$\pu= \pu$
Now divide one gram by one mole:
$\pu= \frac}} = \pu$
Its the same number! Therefore:
$\pu= \pu$
You need to be more careful with your units. The erroneous result is that you are equating a value in amu with a value in grams per mole .
There are two things that routinely mystify science students:
anything to do with amount of substance , the mole, and the Avogadro constant , and
Chemistry Is Everywhere: Sulfur Hexafluoride
On March 20, 1995, the Japanese terrorist group Aum Shinrikyo released some sarin gas in the Tokyo subway system; twelve people were killed, and thousands were injured. Sarin is a nerve toxin that was first synthesized in 1938 . It is regarded as one of the most deadly toxins known, estimated to be about 500 times more potent than cyanide. Scientists and engineers who study the spread of chemical weapons such as sarin would like to have a less dangerous chemical, indeed one that is nontoxic, so they are not at risk themselves.
Figure 4.
Sulfur hexafluoride is used as a model compound for sarin. SF6 has a similar molecular mass as sarin , so it has similar physical properties in the vapour phase. Sulfur hexafluoride is also very easy to accurately detect, even at low levels, and it is not a normal part of the atmosphere, so there is little potential for contamination from natural sources. Consequently, SF6 is also used as an aerial tracer for ventilation systems in buildings. It is nontoxic and very chemically inert, so workers do not have to take special precautions other than watching for asphyxiation.
Figure 5.
Sulfur hexafluoride also has another interesting use: a spark suppressant in high-voltage electrical equipment. High-pressure SF6 gas is used in place of older oils that may have contaminants that are environmentally unfriendly in the accompanying figure).
Origin Of The Concept
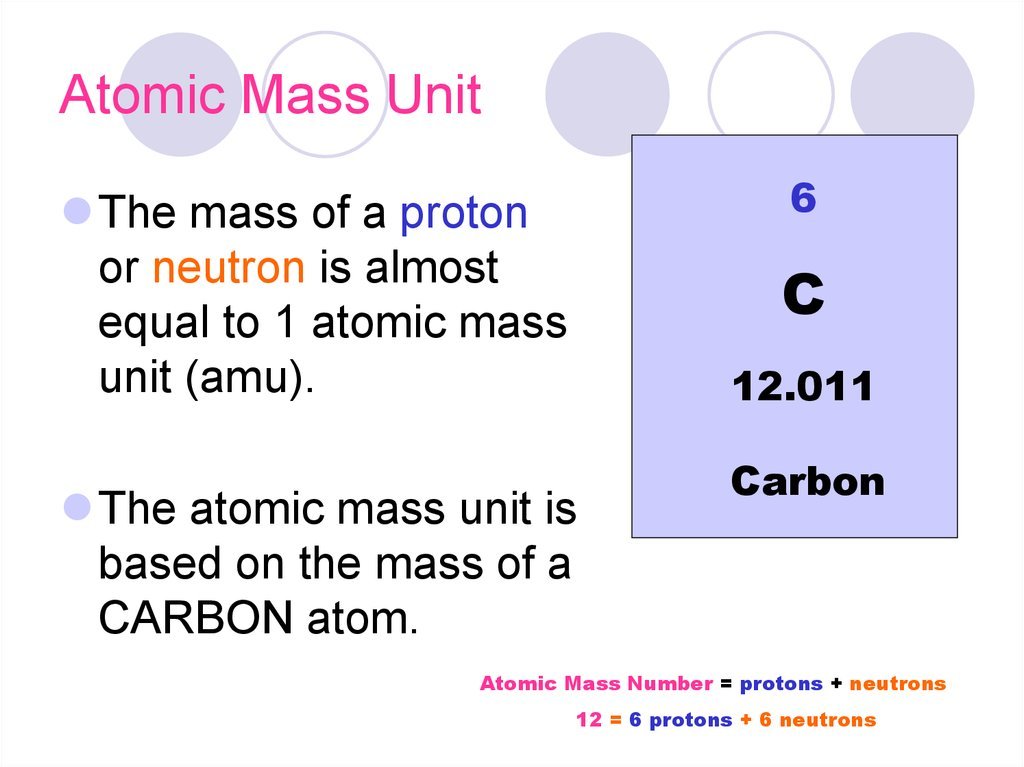
The interpretation of the law of definite proportions in terms of the atomic theory of matter implied that the masses of atoms of various elements had definite ratios that depended on the elements. While the actual masses were unknown, the relative masses could be deduced from that law. In 1803 John Dalton proposed to use the atomic mass of the lightest atom, that of hydrogen, as the natural unit of atomic mass. This was the basis of the atomic weight scale.
For technical reasons, in 1898, chemist Wilhelm Ostwald and others proposed to redefine the unit of atomic mass as 116 of the mass of an oxygen atom. That proposal was formally adopted by the International Committee on Atomic Weights in 1903. That was approximately the mass of one hydrogen atom, but oxygen was more amenable to experimental determination. This suggestion was made before the discovery of the existence of elemental isotopes, which occurred in 1912. The physicist Jean Perrin had adopted the same definition in 1909 during his experiments to determine the atomic masses and Avogadros constant. This definition remained unchanged until 1961. Perrin also defined the mole as an amount of a compound that contained as many molecules as 32 grams of oxygen . He called that number the Avogadro number in honor of physicist Amedeo Avogadro.
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answer Key
Sample Molecular Weight Calculation
The calculation for molecular weight is based on the molecular formula of a compound . The number of each type of atom is multiplied by its atomic weight and then added to the weights of the other atoms.
For example, the molecular formula of hexane is C6H14. The subscripts indicate the number of each type of atom, so there are 6 carbon atoms and 14 hydrogen atoms in each hexane molecule. The atomic weight of carbon and hydrogen may be found on a periodic table.
- Atomic weight of carbon: 12.01
- Atomic weight of hydrogen: 1.01
molecular weight = + so we calculate as follows:
- molecular weight = +
Recommended Reading: What Is An Experimental Study In Psychology
Redefinition Of The Si Base Units
The definition of the dalton was not affected by the 2019 redefinition of SI base units, that is, 1;Da in the SI is still 112 of the mass of a carbon-12 atom, a quantity that must be determined experimentally in terms of SI units. However, the definition of a mole was changed to be the amount of substance consisting of exactly 6.02214076×1023 entities and the definition of the kilogram was changed as well. As a consequence, the molar mass constant is no longer exactly 1;g/mol, meaning that the number of grams in the mass of one mole of any substance is no longer exactly equal to the number of daltons in its average molecular mass.
Read Also: What Is Heuristic In Psychology
Read Also: Segment Addition Postulate Practice Answer Key
Mole A Unit Of Measurement
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A mole is simply a unit of measurement. In fact, it’s one of the seven base units in the International System of Units . Units are invented when existing units are inadequate. Chemical reactions often take place at levels where using grams wouldn’t make sense, yet using absolute numbers of atoms/molecules/ions would be confusing, too. So, scientists invented the mole to bridge the gap between very small and very large numbers.
Here is a look at what a mole is, why we use moles, and how to convert between moles and grams.
Converting Moles To Grams
One of the most common chemistry calculations is converting moles of a substance into grams. When you balance equations, you’ll use the mole ratio between reactants and reagents. To do this conversion, all you need is a periodic table or another list of atomic masses.
Example: How many grams of carbon dioxide is 0.2 moles of CO2?
Look up the atomic masses of carbon and oxygen. This is the number of grams per one mole of atoms.
Carbon has 12.01 grams per mole.Oxygen has 16.00 grams per mole.
One molecule of carbon dioxide contains 1 carbon atom and 2 oxygen atoms, so:
number of grams per mole CO2 = 12.01 + number of grams per mole CO2 = 12.01 + 32.00number of grams per mole CO2 = 44.01 gram/mole
Simply multiply this number of grams per mole times the number of moles you have in order to get the final answer:
grams in 0.2 moles of CO2 = 0.2 moles x 44.01 grams/molegrams in 0.2 moles of CO2 = 8.80 grams
It’s good practice to make certain units cancel out to give you the one you need. In this case, the moles canceled out of the calculation, leaving you with grams.
Recommended Reading: Geometry Segment Addition Postulate Worksheet
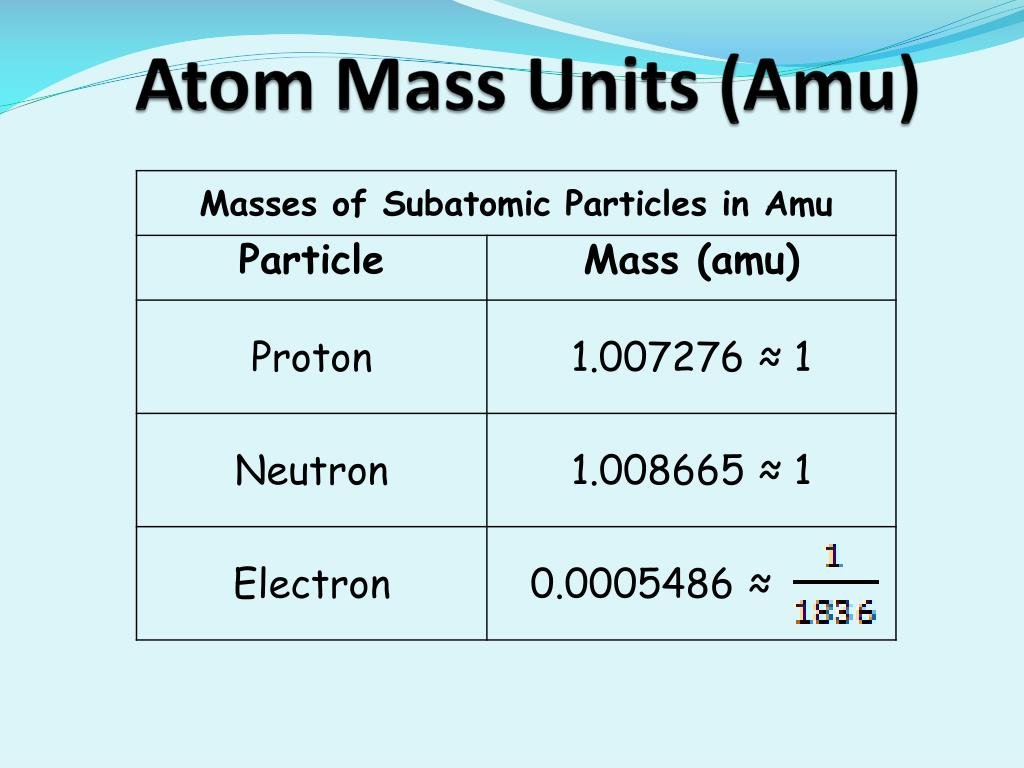
What Is Amu In Chemistry
August 12, 2012, Nathan,
AMU refers to atomic mass unit. ;This particular system of measurement is also referred to as a dalton with the symbol Da. ;As a form of measurement, the AMU is used to indicate the so-called mass of an atom or molecule. ;When talking about the mass of a particular molecule, for example, it simply refers to the total number of protons and neutrons. ;When there are more protons and neurons, the mass and the AMU will also be larger. ;As a measurement unit, the AMU is basically 1/12 of the total mass of a carbon-12 atom. ;The 12 represents the number of protons and neutrons in the carbon molecule. Consequently, 1 atomic mass unit or AMU is also equivalent to one atom of the element hydrogen.
Formula Mass For Covalent Substances
For covalent substances, the formula represents the numbers and types of atoms composing a single molecule of the substance; therefore, the formula mass may be correctly referred to as a molecular mass. Consider chloroform , a covalent compound once used as a surgical anesthetic and now primarily used in the production of the anti-stick polymer, Teflon. The molecular formula of chloroform indicates that a single molecule contains one carbon atom, one hydrogen atom, and three chlorine atoms. The average molecular mass of a chloroform molecule is therefore equal to the sum of the average atomic masses of these atoms. Figure 1 outlines the calculations used to derive the molecular mass of chloroform, which is 119.37 amu.
Figure 1. The average mass of a chloroform molecule, CHCl3, is 119.37 amu, which is the sum of the average atomic masses of each of its constituent atoms. The model shows the molecular structure of chloroform.
Likewise, the molecular mass of an aspirin molecule, C9H8O4, is the sum of the atomic masses of nine carbon atoms, eight hydrogen atoms, and four oxygen atoms, which amounts to 180.15 amu .
Figure 2. The average mass of an aspirin molecule is 180.15 amu. The model shows the molecular structure of aspirin, C9H8O4.
Read Also: Ccl4 Electron Geometry
What Does Amu Stand For Chemistry
We compiled queries of the AMU abbreviation in Chemistry in search engines. The most frequently asked AMU acronym questions for Chemistry were selected and included on the site.
We thought you asked a similar AMU question to the search engine to find the meaning of the AMU full form in Chemistry, and we are sure that the following Chemistry AMU query list will catch your attention.
Unified Atomic Mass Unit
The unified atomic mass unit , or dalton , is a unit of atomic and molecular mass. By definition it is one twelfth of the mass of an unbound carbon-12 atom, at rest and in its ground state.
The relationship of the unified atomic mass unit to the macroscopic SI base unit of mass, the kilogram, is given by Avogadros numberNA. By the definition of Avogadros number, the mass of NA carbon-12 atoms, at rest and in their ground state, is 12 gram . From the latest value of NA follows the latest value of the unified atomic mass unit:
- 1 u 1.660538782 × 1027 kg
Future refinements in Avogadros number by future improvements in counting large numbers of atoms, will give better accuracy of u. It is hoped that in the future the experimental accuracy of Avogadros constant will improve so much that the unified atomic mass unit may replace the kilogram as the SI base unit, see this article.
The unit u is convenient because one hydrogen atom has a mass of approximately 1 u, and more generally an atom or molecule that contains pprotons and nneutrons will have a mass approximately equal to u. The mass of a nucleus is not exactly equal to p + n, because the nuclear binding energy gives rise to a relativistic mass defect.
Recommended Reading: What Is The Molecular Geometry Of Ccl4
Atomic Masses And Atomic Weights
The universal mass unit, abbreviated u , is defined as one-twelfth of the mass of the 12C atom which has been defined to be exactly 12 u. The absolute mass of a 12C atom is obtained by dividing the value 12 by the Avogadro number . The value for the mass of a 12C atom, i.e. the nucleus plus the 6 extranuclear electrons, is thus 1.992 648 × 1023 g. Atomic masses are expressed in units of u relative to the 12C standard. This text uses M to indicate masses in units of u, and m in units of kilograms; m = M/103NA.
In nuclear science it has been found convenient to use the atomic masses rather than nuclear masses. The number of electrons are always balanced in a nuclear reaction, and the changes in the binding energy of the electrons in different atoms are insignificant within the degree of accuracy used in the mass calculations. Therefore the difference in atomic masses of reactants and products in a nuclear reaction gives the difference in the masses of the nuclei involved. In the next chapter, where the equivalence between mass and energy is discussed, it is shown that all nuclear reactions are accompanied by changes in nuclear masses.
If an element consists of n1 atoms of isotope 1, n2 atoms of isotope 2, etc., the atomic fraction x1 for isotope 1 is defined as:
James G. Speight PhD, DSc, PhD, in, 2020
Also Check: How To Find Ksp Chemistry
Examples Of Values Expressed In Atomic Mass Units
- A hydrogen-1 atom has a mass of 1.007 u .
- A carbon-12 atom is defined as having a mass of 12 u.
- The largest known protein, titin, has a mass of 3 x 106 Da.
- AMU is used to differentiate between isotopes. An atom of U-235, for example, has a lower AMU than one of U-238, since they differ by the number of neutrons in the atom.
Don’t Miss: Who Are Paris Jackson’s Biological Parents
How To Convert Grams To Amu
If you want to know the atomic mass of an element, you’ll find it listed under the symbol for that element in the periodic table. The units aren’t included with the mass, but they are understood to be atomic mass units or, more correctly, unified atomic mass units . In macroscopic terms, the number in the periodic table also refers to the weight of a mole of the element in grams. A mole equals Avogadro’s number of atoms.
TL;DR
One AMU is equivalent to 1.66 x 10-24 grams. One gram is equivalent to 6.022 x 1023 AMU.
Main Difference Amu Vs Grams
The terms amu and grams are used to measure the mass of substances. Therefore, grams can be converted into amu units and amu units can be converted into grams as well. Gram is a larger unit when compared to amu, but gram is a smaller unit compared to other units used for the measurement of mass. The term amu stands for atomic mass unit and is used for the measurements of very small substances such as atoms. The amu is the unit that is used to express the atomic mass of a chemical element. The main difference between amu and grams is that amu is used to express the mass in atomic level whereas gram is used as a metric unit of mass.
Also Check: How Many Questions Can You Miss On The Ged Math Test To Pass
How To Find Amu In Chemistry
The absolute masses of the individual atoms of substances are extremely small. The calculation of amu in chemistry is used in order to avoid chemical calculations with the very small numerical values of the absolute atomic masses, a ratio was introduced the relative atomic mass and the absolute masses are compared with this unit of mass.
The relative atomic mass indicates how many times larger the mass of an atom is than the atomic mass unit.
Formula symbol: Ar
Unit: 1
The relative atomic mass of an element can be read from the periodic table of the elements.
The mass of individual atoms of an element is so small that it cannot be determined directly. However, one can determine mass relations between the atoms of different elements.
In the electrolysis of water, hydrogen and oxygen are obtained in a mass ratio of 1: 7.936, so the oxygen atom is 15.872 times as heavy as a hydrogen atom. Similarly, one can determine relations for the other elements through quantitative analysis of compounds and then create a mass scale of the elements using an arbitrarily chosen mass unit.
One could have established the mass of the hydrogen atom as the lightest element as a unit of mass. However, since almost all elements form oxygen compounds, for practical reasons the mass of 1/16 of the mass of oxygen was initially chosen as the unit of mass, so that the mass of hydrogen is approximately 1.
1 / 6.0221367 * 1023 = 1.6605402 * 10-24 g = 1.6605402 * 10-27 kg
How Is Amu Determined
For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number. This is because each proton and each neutron weigh one atomic mass unit . By adding together the number of protons and neutrons and multiplying by 1 amu, you can calculate the mass of the atom.
You May Like: Who Is The Biological Father Of Paris Jackson
What Is One Amu In Grams
A mole of carbon-12 atoms weighs 12 grams, and there are 6.022 x 1023 atoms in a mole. Dividing 12 grams by this incredibly large number of atoms tells us that one carbon-12 atom weighs 1.99 x 10-23 grams. Since a carbon atom weighs 12 AMU, one AMU is equivalent to 1.66 x 10-24 grams. Conversely, one gram is equivalent to 6.022 x 1023 AMU, which is Avogadro’s number.
Related Articles