What Is Theoretical Yield In Chemistry
The theoretical yield definition of a chemical process is the amount of product that will theoretically be generated by a chemical reaction under “perfect” conditions.Perfect conditions include, but are not limited to: fully consuming the available reactant, no impurities in the reactants, no production ofunexpected byproducts which can reduce the yield, and no losses due to any measurement, processing, and handling of reactants or products.
Yield Calculations Chemistry Tutorial
- Yield is the mass of product formed in a chemical reaction.
- Actual yield is the mass of product formed in an experiment or industrial process.
- Theoretical yield is the mass of product predicted by the balanced chemical equation for the reaction.
- Percentage yield = × 100
- Optimum yield is the best possible yield achieved for a set of given reaction conditions.
- For a chemical reaction which goes to completion:
- For a chemical reaction at equilibrium:
- For a chemical reaction at equilibrium, actual yield can be affected by factors such as:
actual yield = theoretical yield
actual yield < theoretical yield
temperature
pressure and volume
Please do not block ads on this website. No ads = no money for us = no free stuff for you!
Reasons For Getting Lower Percentage Yield
Also Check: My.hrw Answers
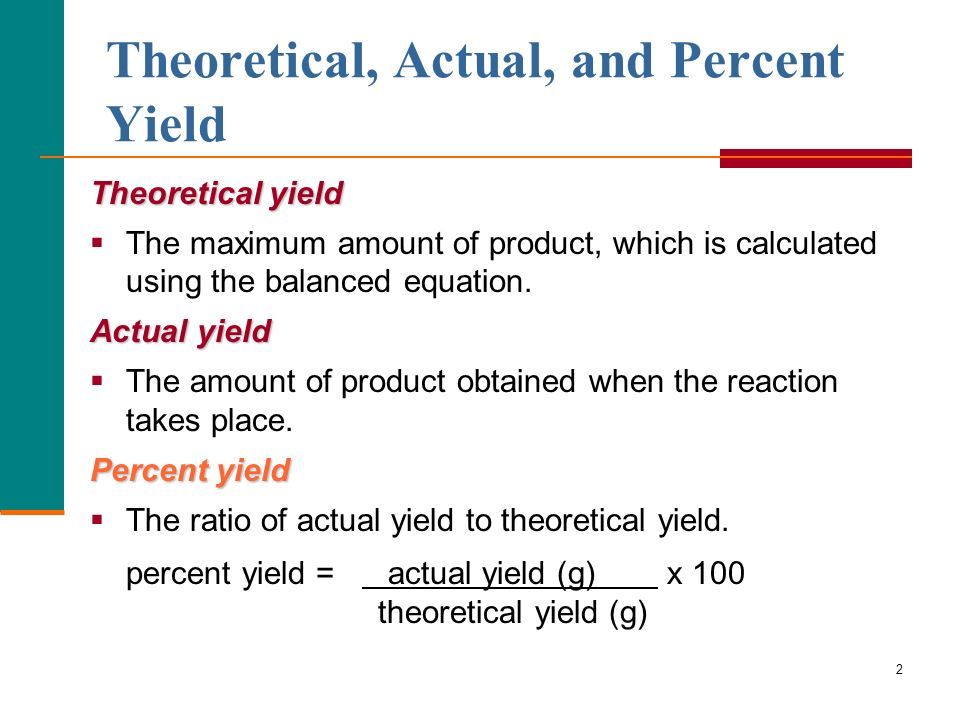
What Is The Formula To Calculate Percent Yield
Percent yield = x 100Where,
-
Actual yield is the amount of product obtained from a chemical reaction via an experiment.
-
Theoretical yield is the amount of product obtained mathematically from the stoichiometric or balanced equation.
Note that the units for both the actual and theoretical yield need to be the same, that is, moles or grams.
Percent Yield Definition And Formula
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by theoretical yield multiplied by 100%. If the actual and theoretical yield are the same, the percent yield is 100%. Usually, percent yield is lower than 100% because the actual yield is often less than the theoretical value. Reasons for this can include incomplete or competing reactions and loss of sample during recovery. It’s possible for percent yield to be over 100%, which means more sample was recovered from a reaction than predicted. This can happen when other reactions were occurring that also formed the product. It can also be a source of error if the excess is due to incomplete removal of water or other impurities from the sample. Percent yield is always a positive value.
Also Known As: percentage yield
Recommended Reading: Kuta Software Infinite Algebra 2 Rationalizing Imaginary Denominators
Procedure To Calculate A Reactions Theoretical Yield
The first thing we need to consider when evaluating a chemical reaction is the chemical equation. This expression represents what happens in reality. Since matter cant be created or destroyed, the number of atoms of each species on both sides of the equation must be the same. Otherwise, it would mean some atoms appear or disappear during the chemical reaction, which is simply not the case.
This correction of a chemical equation is called balancing the chemical reaction. Since we cant alter the subscripts of a chemical compound, which means we would alter the compound itself, we need to balance a chemical equation through its stoichiometric coefficients. Lets say you are presented with the following chemical equation for the combustion of propane:
On the left hand side you have three carbon atoms, while on the right side you only have one. To solve this, you need to add a stoichiometric coefficient of 3 to carbon dioxide on the right hand side. This way, the three initial carbon atoms are preserved. If you do so, you end up with another discrepancy, though: you have a total of + 1 = 7 oxygen atoms on the right side, while, on the left side, you have a total of 5 x 2 = 10 oxygen atoms. To solve this, you need to add a stoichiometric coefficient of 4 to water. This way, you end up with + 4 = 10 oxygen atoms on the right side, and so mass if preserved. The balanced chemical equation is then:
Calculate Reactant Needed To Make A Set Amount Of Product
This strategy can be slightly modified to calculate the amount of reactants needed to produce a set amount of product. Let’s change our example slightly: How many grams of hydrogen gas and oxygen gas are needed to produce 90 grams of water?
We know the amount of hydrogen needed , but to do the calculation:
grams reactant = grams product x x x
For hydrogen gas:
grams H2= 90 grams H2O x x x
grams H2= grams H2 grams H2= 10 grams H2
This agrees with the first example. To determine the amount of oxygen needed, the mole ratio of oxygen to water is needed. For every mole of oxygen gas used, 2 moles of water are produced. The mole ratio between oxygen gas and water is 1 mol O2/2 mol H2O.
The equation for grams O2 becomes:
grams O2= 90 grams H2O x x x
grams O2= grams O2
grams O2= 80 grams O2
To produce 90 grams of water, 10 grams of hydrogen gas and 80 grams of oxygen gas are needed.
Theoretical yield calculations are straightforward as long as you have balanced equations to find the mole ratios needed to bridge the reactants and the product.
You May Like: Simplifying Radicals Worksheet Kuta
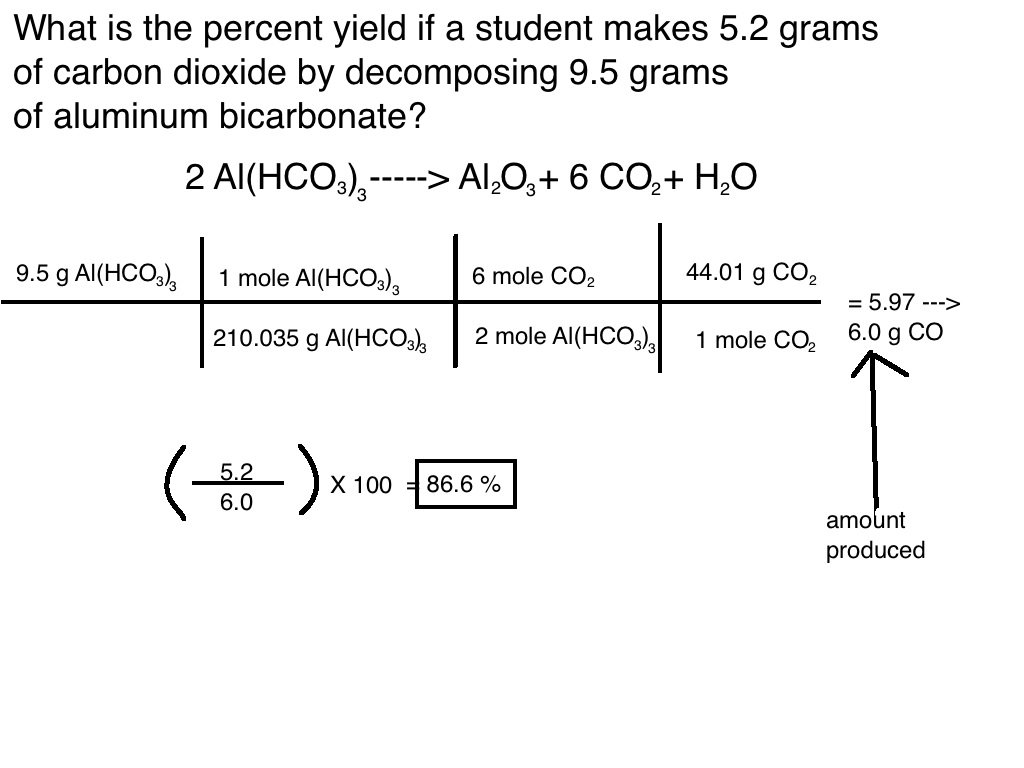
Find The Percentage Yield
If you actually carry out this reaction in a lab, you will be able to find the actual yield of the reaction. Based on that value, you can find the percentage yield by using the ratio of the actual yield and the theoretical yield. The formula for calculating the percent yield is:
Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100%
Lets assume that you obtained an actual yield of 8.50 grams. Then, the percent yield would be:
Percentage yield of NaCl = 8.50 grams ÷ 9.93 grams × 100%
Percentage yield of NaCl = 85.59%
Since the value of actual yield is usually less than the theoretical yield, the percentage yield is always less than 100%.
What Is Theoretical Yield
Theoretical yield refers to the maximum possible mass of a product that can be made as a result of a chemical reaction. Using stoichiometry, chemists are able to determine precisely how much should result, however actual chemical reactions almost never occur exactly as these formulae predict.
There are unavoidable experimental errors, side reactions, or incomplete chemical reactions in the real world. Companies that deal in chemical production of any sort have a huge interest in eliminating these factors as much as possible to achieve a yield as close to the theoretical yield as possible.
Read Also: Lewis Structures And Molecular Geometry Models Of Covalent Bonding Lab Answers
Determine The Limiting Reagent
Calculate the molar mass of all the compounds in the chemical reaction. The molar mass is the sum of the atomic mass of each atom in a compound. For example, the molar mass of water is 18 grams: 2 grams of hydrogen plus 16 grams of oxygen.
Divide the grams of the compounds by their molar masses. This will give you the number of moles of each compound in the experiment. For example, if you initially used 36 grams of water, 36 divided by 18 grams per mole yields 2 moles of water.
Compare the moles of reactants in your experiment to the theoretical number of moles. For example, consider the chemical reaction 2F2 + 2H2O => 4HF + O2, in which “F” is fluorine, “HF” is hydrogen fluoride and “O2” is oxygen. In this case, you want equal moles of F2 and H2O. If you have 2 moles of H2O and 2.3 moles of F2, however, you have more than enough F2 to complete the reaction. Hence, H20 is the limiting reagent.
Theoretical Yield And Percent Yield
The world of pharmaceutical production is an expensive one. Many drugs have several steps in their synthesis and use costly chemicals. A great deal of research takes place to develop better ways to make drugs faster and more efficiently. Analysis of how much of a compound is produced in any given reaction is an important part of cost control.
Also Check: Elton John Kids Adopted
How To Calculate Percent Yield
As you may have guessed from the percent yield equation above, if you want to know how to calculate the percent yield, you need two things, your experimental yield and the theoretical yield . Let’s assume you have both values how to find the percent yield?
There you go, not too complicated right! Or you could use our percent yield calculator to calculate it easily and quickly. A note about the values obtained a value above 100% is possible but is due to solvent being present in the sample as well as your product. Dry your product thoroughly and re-weight to get the true percent yield. Also, a value of 100% is impossible to achieve there will always be some molecules that do not react or that are left on the side of the glassware. A value of 70% or higher is acceptable!
The Limiting Reagent And Excess Reagent
In a chemical reaction, you have both limiting reagents and excess reagents. The limiting reagents are the lesser in numbers and determine how much of a product will result. Therefore, limiting reagents often equals product. Excess reagents are the chemical or chemicals left over, the by-products of a reaction. The limiting reagent is important because for higher effectivity you want a balanced reaction. The ratio of limiting reagent, or limiting reactant and excess reagent, should be as close to 1:1 as possible. The more balanced the solution the less waste results.
You May Like: Geography Movement Definition
Examples On Percent Yield
Example 1: During a chemical reaction, 0.5 g of product is made. The maximum calculated yield is 1.6 g. What is the percent yield of this reaction?
Solution:
We know that according to Percent Yield Formula,
Percentage yield = × 100%
= 0.5/1.6× 100%
= 31.25%
Therefore, the percentage yield of this reaction is 31.25%.
Example 2: During a chemical reaction 1.8 g of product is made. The maximum calculated yield is 3.6 g. What is the percent yield of this reaction?
Solution:
We know that according to Percent Yield Formula,
Percentage yield = × 100%
= 1.8/3.6× 100%
= 50%
Therefore, the percentage yield of this reaction is 50%.
Example 3: If the percentage yield is 45% with the theoretical yield as 4g, what would the actual yield be? Calculate using the percentage yield formula.
Solution:
Using the percentage yield formula,
Percentage yield = × 100%
45 = Actual yield/4 × 100
Balance The Chemical Equation
The chemical equation for the reaction is:
Na + Cl2 NaCl
The first step is to balance the chemical equation. It involves adding numerical coefficients called the stoichiometric coefficients before the reagents and products, so that the number of atoms or molecules on the reactant side and the product side become equal. In the given problem, adding numeral 2 before Na and NaCl, balances the total number of sodium and chlorine atoms on both sides of the equation. Now the balanced equation is:
2Na + Cl2 2NaCl
You May Like: Geometry Escape Challenge A Answer Key
How To Find Theoretical Yield
To determine the theoretical yield of any chemical reaction, multiply the number of moles by the molecular weight. Theoretical yield will be calculated in grams because it uses the theoretical yield equation and it is the amount of the expected product. This makes calculating theoretical yield easy.
Now we will solve example with theoretical yield formula to make it more clear. To learn about grams & moles and to calculate their values, use grams to moles calculator.
What Is The Formula For Percent Yield
Conversely, if you made a mistake and you poured the whole mixture on the floor or down the drain, then youd have a percent yield of 0%. This may seem confusing to a lot of people which is why its better to use the percent yield calculator to perform the calculations.
Still, if you want to do the calculation by hand, you should use the percent yield equation:
percent yield = * 100
For this equation, you must know two out of the three valuables. But its a flexible formula which means that it doesnt matter which variables you know. Either way, you can still use the equation by rearranging it according to the missing value.
In the same way, if you need to find the value of the theoretical percent yield, you can use a theoretical yield calculator.
Also Check: Geometry Dash 2.0 Sneak Peek
Calculating Percent Yield Example
Now that we know the steps to calculate percent yield, lets walk through an example:
Use the balanced chemical reaction below. If 40.00 g of Acetylene and 65.00 g of Oxygen are used, and 25.00 g of water are produced, what is the percent yield?
First step is to find limiting reagent& theoretical yield of water:
Using dimensional analysis on both reagents, acetylene is found to produce a lower amount of product than oxygen because of this acetylene is our limiting reagent.
27.67g is our theoretical yield. The last step is to plug our numbers into the percent yield equation.
Our percent yield is 90.35%.
For more example questions to try, click here!
Examples Of Calculating Percent Yield
Percent Yield Example 1:
Percent Yield Example 2:
A major healthcare company produces hydrogen peroxide , a chemical compound made up of hydrogen and oxygen used to clean out small cuts and scrapes. The company wants to conduct an experiment concerned with the decomposition of hydrogen peroxide to understand their processes effectiveness.
The theoretical yield of the hydrogen peroxide decomposition is 54.3. After measuring the actual yield during the reaction, it is 23.7. The companys chemist puts this information in the percent yield formula in the appropriate places.
X 100 = Percent Yield.
The chemist divides 23.7 by 54.3 to arrive at the unrefined percent yield value of 0.436.
He multiples this decimal value by 100 to get the actual percent yield.
0.436 X 100 = 43.6
The percent yield is 43.6%.
What it means: This percent yield result isnt nearly as encouraging as the one from the previous example. As stated earlier, any percent yield over 60% is generally considered to be positive productivity. 43.6% is clearly under this standard. However, this value isnt necessarily terrible.
It isnt until a percent yield value is lower than 40% that its thought of as poor and unacceptable in terms of efficiency. While the percent yield result of 43.6% is cutting it very close to being negative, it is still considered acceptable.
Read Also: What Does Span Mean In Linear Algebra
Calculate The Percentage Yield:
The percent yield is simply the actual yield divided by theoretical yield multiplied by 100. Actual yield is the amount of product you actually got while theoretical is the maximum possible yield. Be sure that actual and theoretical yields are both in the same units so that units cancel in the calculation.
Example:
In the reaction below we calculated a theoretical yield of 1 mol, and obtained an actual yield of 0.55 mol. What is the percent yield?
Document Actions
Bookmarking Save And Share Results
The tool is designed so you can flip between different parts of a problem set. We recommend you bookmark it so you can refer back to it.You can also by hitting calculate and copying the URL for this page. When your study partneropens up the URL, they will see your calculations. It’s easy share & save results via email.
You also have the option of saving links to the calculations in your research notes files, so you can quickly re-open or check them later.Again – hit calculate first so the URL is updated with your most recent changes. Then copy and save the url.
Read Also: Unit 1 Geometry Basics Homework 2 Segment Addition Postulate Answer Key
Find The Theoretical Yield
We have found that Na is the limiting reagent in the reaction, and that for 0.17 moles of Na, 0.17 moles of NaCl are produced. Therefore, the theoretical yield of NaCl in moles is 0.17 moles.
But this value is in terms of moles. In the given problem, we need to find out how many grams of NaCl would be produced in the reaction.
We can convert the value from moles to grams by multiplying it with the molar mass of NaCl, which is equal to 58.44 g/mole.
Theoretical yield of NaCl in grams = theoretical yield in moles × molar mass of NaCl
Theoretical yield of NaCl in grams = 0.17 moles of NaCl × 58.44 g/mole
Theoretical yield of NaCl in grams = 9.93 grams