Other Words From Concentration
Top Definitions Quiz Related Content Examples British Medical Scientific This shows grade level based on the words complexity. / kn sntre n /This shows grade level based on the words complexity. nounthe act of concentrating the state of being concentrated. exclusive attention to one object close mental application. something concentrated: a concentration of stars. Military. the assembling of military or naval forces in a particular area in preparation for further operations. a specified intensity and duration of artillery fire placed on a small area. the focusing of a students academic program on advanced study in a specific subject or field. Chemistry. a measure of the amount of dissolved substance contained per unit of volume. Also called memory. Cards. a game in which all 52 cards are spread out face down on the table and each player in turn exposes two cards at a time and replaces them face down if they do not constitute a pair, the object being to take the most pairs by remembering the location of the cards previously exposed.
Video advice: The Science of Thinking
How the brain works, how we learn, and why we sometimes make stupid mistakes.
How To Dilute Solutions
This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been viewed 360,701 times.
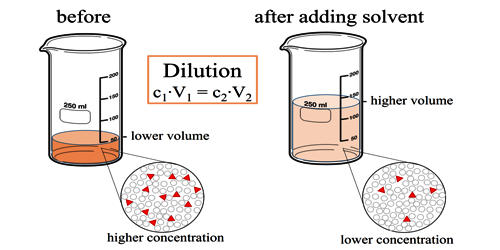
Dilution is the process of making a concentrated solution less concentrated. There are a variety of reasons why one might want to perform a dilution. For example, biochemists dilute solutions from their concentrated form to create new solutions for use in their experiments. As a more casual type of dilution, bartenders often dilute hard liquor with a soft drink or juice to make a cocktail more palatable. For diluting solutions in lab experiments, the formal formula for calculating a dilution is C1V1 = C2V2, where C1 and C2 represent the concentrations of the initial and final solutions, respectively, and V1 and V2 represent their volumes.
Definitions And Examples Of How To Calculate Wt% Molarity And How To Prepare Dilutions
A solution is in chemistry defined as:
- A homogeneous mixture composed of two or more substances.
- The process by which a gas, liquid, or solid is dispersed homogeneously in a gas, liquid, or solid without chemical change.
The substance in which another substance dissolves is called the solvent, while the dissolved substance is called the solute.
The solution assumes the characteristics of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. From this, the solute is usually the component of a solution present in the lesser amount.
Solubility is the ability of a solute to dissolve in a solvent. The solubility of a substance in another is not unlimited, and how much solute you can solve in a solvent varies a lot. Tabulated values of solubility refer to max grams of solute in a given amount of the solvent.
- solubility is dependent on temperature. In general, but not without expectations, solubility increases with temperature
- smaller particles dissolves faster than larger particles
- a solid dissolves faster if the mixture is stirred or shaken
A saturated solution contains the maximum amount of solute in the solvent, given by the solubility.
Examples of common solutions:
- tea with sugar – a solution of sugar in hot water
- seawater – a solution of salt in water
The concentration of a solute in a solution is can be given as
Don’t Miss: Is Michael Jackson The Biological Father Of His Kids
What Is A Solution
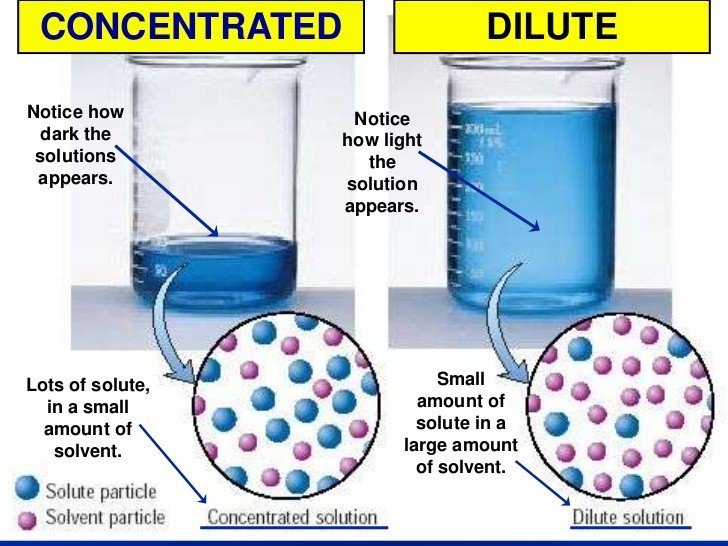
Before you can make a simple dilution, it is a good idea to understand the terminology since some of the words sound similar. A solution is a liquid mixture where a smaller amount of a substance called a solute is mixed into a larger amount of a solvent like water. A solution with a great deal of solute is concentrated while a solution with a smaller amount of solute is dilute.
Sometimes you must use a concentrated solution and add more solvent to create a simple dilution. To visualize, household bleach is a solution that contains sodium hypochlorite and water. This solution is much too concentrated to use directly from the bottle, so you add water in a spray bottle, bowl or the basin of the washing machine to create a simple dilution of bleach.
What Formula Is M1v1 M2v2
You can solve for the concentration or volume of the concentrated or dilute solution using the equation: M1V1 = M2V2, where M1 is the concentration in molarity of the concentrated solution, V2 is the volume of the concentrated solution, M2 is the concentration in molarity of the dilute solution (after
Recommended Reading: Does Michael Jackson Have A Biological Son
Starting With Final Volume
If your simple dilution requires a more precise final volume, you should first determine how many total parts your final solution will contain. In a 1:4 ratio, there are five total parts . You can then divide the end volume by the total parts to determine the volume of one part. For example, if you know you need 40 ounces of that 1:4 bleach dilution, you can divide 40 ounces by 5 parts and find that each part is 8 ounces. Using simple subtraction, you know you will need 8 ounces of bleach and 32 ounces of water.
Whether you are making simple dilutions to use in your home or in a laboratory, understanding dilution ratios is an invaluable skill.
Related Articles
What Do You Mean By Dilution In Law
The use of a mark or trade name in commerce sufficiently similar to a famous mark that by association it reduces, or is likely to reduce, the publics perception that the famous mark signifies something unique, singular or particular. Dilution is comprised of two principal harms: blurring and tarnishment.
Also Check: Is Paris Jackson Michael Jackson Biological Daughter
Examples Of Concentrated Solutions
12 M HCl is more concentrated than 1 M HCl or 0.1 M HCl. 12 M hydrochloric acid is also called concentrated sulfuric acid because it contains a minimum amount of water.
When you stir salt into water until no more dissolves, you make a concentrated saline solution. Similarly, adding sugar until no more dissolves produces a concentrated sugar solution.
Dilutions Of Stock Solutions
Imagine we have a salt water solution with a certain concentration. That means we have a certain amount of salt dissolved in a certain volume of solution. Next, we will dilute this solution. This is done by adding more water, not more salt:
Before Dilution and After Dilution
The molarity of solution 1 is
and the molarity of solution 2 is
rearrange the equations to find moles:
What stayed the same and what changed between the two solutions? By adding more water, we changed the volume of the solution. Doing so also changed its concentration. However, the number of moles of solute did not change. So,
where
- \ and \ are the concentrations of the original and diluted solutions
- \ and \ are the volumes of the two solutions
Preparing dilutions is a common activity in the chemistry lab and elsewhere. Once you understand the above relationship, the calculations are simple.
Suppose that you have \ of a \ solution of \. You dilute the solution by adding enough water to make the solution volume \. The new molarity can easily be calculated by using the above equation and solving for \.
The solution has been diluted by one-fifth since the new volume is five times as great as the original volume. Consequently, the molarity is one-fifth of its original value.
Example \: Diluting Nitric Acid
Solution
Read Also: Unit 1 Test Study Guide Geometry Basics Gina Wilson
Which Is The Correct Formula For The V2 Equation
V2 = Final volume of solution Put together, the equation translates to: the starting concentration multiplied by the starting volume is equal to the final concentration multiplied by the final volume. Basically, if you have three of the four components of the equation then you can use these within the formula to calculate the unknown component.
What does C1V1 C2V2 stand for? C1V1 = Concentration/amount and Volume C2V2 = Concentration/amount and Volume How is dilution concentration calculated? Most commonly, a solution s concentration is expressed in terms of mass percent, mole fraction, molarity, molality, and normality. When calculating dilution factors, it is important that the units of
Problem Solving : Dilution Factor
The Problem:
The site of a meteorite which fell to Earth millions of years ago is suspected of having enough iron in it to make it worthwhile trying to mine it.Jo the Geologist has taken a sample of the meteorite to Chris the Chemist to determine how much iron is present.Chris has dissolved 10.00 grams of the meteorite sample in 5.00 L of acid to produce a stock solution. Chris ran a trial of the analytical procedure on the stock solution, but found the solution was too concentrated to be used.So Chris prepared a 1:250 dilution of the sample solution, and, after running the analytical procedure successfully, found the solution contained 1.62 x 10-5 mol L-1 Fe2+.What is the mass of iron in the meteorite sample?
Solving the Problem
Using the StoPGoPS model for problem solving:
| STOP! |
You May Like: My Hrw Answers Algebra 1
Chemistry Is Everywhere: Preparing Iv Solutions
In a hospital emergency room, a physician orders an intravenous delivery of 100 mL of 0.5% KCl for a patient suffering from hypokalemia . Does an aide run to a supply cabinet and take out an IV bag containing this concentration of KCl?
Not likely. It is more probable that the aide must make the proper solution from an IV bag of sterile solution and a more concentrated, sterile solution, called a stock solution, of KCl. The aide is expected to use a syringe to draw up some stock solution and inject it into the waiting IV bag and dilute it to the proper concentration. Thus the aide must perform a dilution calculation.
If the stock solution is 10.0% KCl and the final volume and concentration need to be 100 mL and 0.50%, respectively, then it is an easy calculation to determine how much stock solution to use:
V1 = V1 = 5 mL
Of course, the addition of the stock solution affects the total volume of the diluted solution, but the final concentration is likely close enough even for medical purposes.
Medical and pharmaceutical personnel are constantly dealing with dosages that require concentration measurements and dilutions. It is an important responsibility: calculating the wrong dose can be useless, harmful, or even fatal!
How To Mix One Part Solution To Four Parts Water
From household cleaners to laboratory samples, simple dilutions are all around you. Learning how to use dilution ratios to make dilutions from concentrated solutions or samples is a valuable skill both inside and outside the chemistry lab.
TL DR
A 1:4 dilution ratio means that a simple dilution contains one part concentrated solution or solute and four parts of the solvent, which is usually water. For example, frozen juice that requires one can of frozen juice plus four cans of water is a 1:4 simple dilution.
Don’t Miss: Kim Kardashian’s Biological Father
Examples Of Dilute In A Sentence
dilutediluteddilutingdilutedilutedilute The New Yorkerdilute Los Angeles Timesdilute The Hollywood Reporterdilute WSJdilute baltimoresun.comdilute orlandosentinel.comdilute Timedilute New York Timesdilute Science | AAASdilute Houston Chronicledilute NOLA.comdilutesacbeedilutecleveland.comdiluteThe Seattle TimesdiluteThe Mercury NewsdiluteNew York Times
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘dilute.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
How Is Dilution Concentration Calculated
Most commonly, a solution s concentration is expressed in terms of mass percent, mole fraction, molarity, molality, and normality. When calculating dilution factors, it is important that the units of volume and concentration remain consistent. Dilution calculations can be performed using the formula M1V1 = M2V2.
Don’t Miss: Who Is The Biological Father Of Paris Jackson
What Does Concentration Mean In Science For Kids
Supplement. In biochemistry, the term concentration refers to the measure of the amount of a sub-component in a solution. For instance, the concentration of a solute in a solution pertains to the ratio of the mass or volume of the solute to the mass or volume of the solvent.
The phrase concentration means the quantity of ingredients or parts with regards to another ingredients or parts. A good example of concentration is the quantity of salt to water inside a saltwater solution. Concentration is understood to be close mental focus on something. Concentration Definition. In chemistry, concentration refers back to the quantity of an ingredient inside a defined space. Another definition is the fact that concentration is the number of solute in a strategy to either solvent or total solution. Concentration is generally expressed when it comes to mass per unit volume. Furthermore, whats concentration in science fifth grade? may be the solid material that dissolves within the solvent. The home that substances have of dissolving in solvents, like the solubility of salt in water. Concentration. The quantity of material dissolved inside a way of measuring liquid . Beside above, whats concentration in science for children? In chemistry, concentration is the amount of an ingredient is combined with another substance.
Starting With The Solute
The first option works best when you know precisely how much solute or concentrated solution you have or want to use. For example, to make a simple dilution using a 1:4 dilution ratio with a 10 mL sample in a laboratory, you know that one part equals your 10 mL sample. If you multiply that one part by four parts, you know that you should add 40 mL of water to your sample, resulting in a 1:4 ratio .
This strategy also works well for making a simple dilution when your end volume doesnt really matter. For example, if you are making a dilution of bleach for household cleaning, you can quickly mix one part bleach with four parts water to make your 1:4 dilution ratio.
Don’t Miss: Geometry Dash Demon Key Hack
What Is A Dilute Solution
A dilute solution has a low concentration of the solute compared to the solvent. The opposite of a dilute solution is a concentrated solution, which has high levels of solute in the mixture.
To achieve a dilute solution, more solvent is simply added without adding any more solute into the original mixture. The solution is then stirred to mix the two ingredients thoroughly. This ensures that all parts of the mixture have the same composition. The chemicals that can be diluted are gases, vapors and liquids. Solutions are diluted and can be monitored to attain a desired level of concentration.
What Is A Dilution Ratio
When you make a simple dilution that contains one part concentrated solution and four parts water as a solvent, you are using a 1:4 dilution ratio. This means there are five total parts in the diluted solution you have in the end. There are two simple ways to figure out how much solute and solvent you will need: measuring parts based on the amount of solute you have or measuring parts using your intended final volume.
Recommended Reading: Abiotic Biology
What Does Concentration Mean Science
Concentration. In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar concentration has variants such as normal concentration and osmotic concentration.
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar concentration has variants such as normal concentration and osmotic concentration.
Video advice: The art of focus a crucial ability
How do you bring out the best in yourself? According to Christina Bengtsson \r
Concentration If you have great powers of concentration, that means youre able to focus all your attention on the matter at hand. Concentration can also refer to something thats clustered together or to the density or strength of a solution.
When Concentrated Becomes Confusing

While the concept of concentration is straightforward when a solid solute is dissolved into a liquid solvent, it can be confusing when mixing gases or liquids because it’s less clear which substance is the solute and which is the solvent.
Absolute alcohol is considered to be a concentrated alcohol solution because it contains a minimum amount of water.
Oxygen gas is more concentrated in air than carbon dioxide gas. The concentration of both gases could be considered versus the total volume of air or with respect to the “solvent” gas, nitrogen.
Also Check: Redken Acidic Bonder Vs Olaplex
Why Dilution Is Important
Dilution of solutions helps us in many ways. During titration, solutions are diluted so there is a larger volume of solution available. This allows the students to conduct multiple titrations without the fear of running out the solution. Solutions are also diluted because acids, at higher concentrations, are very corrosive to the skin and can have permanent effects. So, for safety reasons as well, solutions are diluted.
Question
25 cm3of KmnO4 at the concentration of 0.1 mol/dm3 is pipetted and added to the beaker. That 25 cm3 is diluted with water till the volume in the beaker is 100 cm3. Find the concentration of KMnO4 in the beaker.
Solution
The first statement indicates that KmnO4 had the original concentration of 0.1 mol/dm3. This can also be written as per 1 dm3 of water, there are 0.1 moles of KMnO4. By using the
ratio method, we can find out the number of moles in the 25 cm3sample that was pipetted out.
| Moles |
X = /1000
X = 0.0025 moles
0.0025 moles of KMnOwere taken out when 25 cm3of the solution was pipetted out.
Then, the 25 cm3was made into 100 cm3by adding water. As we discussed earlier, adding water only makes the concentration and the volume change. The moles and the mass of the solute remain as is. So, the 100 cm3will have the same number of moles as the 25 cm3 that was pipetted out. Now, we know that 100 cm3will have 0.0025 moles of KMnO4 in it, we can calculate its concentration using the ratio method.
| Moles | |
| x | 1000 |
X = /100