The Autoionization Of Water
- To understand the autoionization reaction of liquid water.
- To know the relationship among pH, pOH, and \.
As you learned previously acids and bases can be defined in several different ways ). Recall that the Arrhenius definition of an acid is a substance that dissociates in water to produce \ ions , and an Arrhenius base is a substance that dissociates in water to produce \ ions. According to this view, an acidbase reaction involves the reaction of a proton with a hydroxide ion to form water. Although Brønsted and Lowry defined an acid similarly to Arrhenius by describing an acid as any substance that can donate a proton, the BrønstedLowry definition of a base is much more general than the Arrhenius definition. In BrønstedLowry terms, a base is any substance that can accept a proton, so a base is not limited to just a hydroxide ion. This means that for every BrønstedLowry acid, there exists a corresponding conjugate base with one fewer proton. Consequently, all BrønstedLowry acidbase reactions actually involve two conjugate acidbase pairs and the transfer of a proton from one substance to another . In contrast, the Lewis definition of acids and bases, focuses on accepting or donating pairs of electrons rather than protons. A Lewis base is an electron-pair donor, and a Lewis acid is an electron-pair acceptor.
| Definition | |
|---|---|
| electron-pair acceptor | electron-pair donor |
What Does The Brackets Around And Represent And What Does It Do
In this context, the brackets means #”concentration”#
It is tempting to assume that #HO^-# and #H_3O^+# are actual species in aqueous species. As far as anyone knows these species are CLUSTERS of water molecules LESS or PLUS a proton…
And so or …or something…we use the #HO^”/”H_3O^+# as a label of convenience…the acidium species is a water cluster associated with an extra proton, and the hydroxide species is a water cluster LESS a proton. And if you play rugby think of #H^+# as the ball in a maul.
But certainly, when we do acid-base titrations, we can find the equivalence of acids and bases, straightforwardly and quantitatively, using very simple equipment, calibrated burettes, and standard indicatos.
And we use the brackets to designate a concentration term: #=10^# under standard conditions. And for simplicity, when we see ##
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie; no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Don’t Miss: What Is An Example Of Movement In Geography
Ksp Chemistry: Complete Guide To The Solubility Constant
Are you learning chemistry but dont quite understand the solubility product constant or want to learn more about it? Not sure how to calculate molar solubility from $K_s_p$? The solubility constant, or $K_s_p$, is an important part of chemistry, particularly when youre working with solubility equations or analyzing the solubility of different solutes. When you have a solid grasp of $K_s_p$, those questions become much easier to answer!
In this $K_s_p$ chemistry guide, well explain the $K_s_p$ chemistry definition, how to solve for it , which factors affect it, and why its important. At the bottom of this guide, we also have a table with the $K_s_p$ values for a long list of substances to make it easy for you to find solubility constant values.
The Base Dissociation Constant
Historically, the equilibrium constant Kb for a base has been defined as the association constant for protonation of the base, B, to form the conjugate acid, HB+.
B + H_2O \leftrightharpoons HB^+ + OH^-
As with any equilibrium constant for a reversible reaction, the expression for Kb takes the following form:
K_ = \frac
Kb is related to Ka for the conjugate acid. Recall that in water, the concentration of the hydroxide ion, , is related to the concentration of the hydrogen ion by the autoionization constant of water:
K_W=
Rearranging, we have:
= \frac}
Substituting this expression for into the expression for Kb yields:
K_ = \frac} = \frac}}
Therefore, for any base/conjugate acid pair, the following relationship always holds true:
K_W=K_aK_b
Taking the negative log of both sides yields the following useful equation:
pK_a+pK_b=14
In actuality, there is no need to define pKb separately from pKa, but it is done here because pKb values are found in some of the older chemistry literature.
Also Check: When Was Geometry Dash Made
How Do You Calculate Ka And Kb In Chemistry
4.3/5KaKbKa and KbKa and Kbabout it here
Solve the equation for Kb by dividing the Kw by the Ka. You then obtain the equation Kb = Kw / Ka. Put the values from the problem into the equation. For example, for the chloride ion, Kb = 1.0 x 10^-14 / 1.0 x 10^6.
Beside above, what are the KA and KB equations? Ka and Kb values measure how well an acid or base dissociates. Higher values of Ka or Kb mean higher strength. General Ka expressions take the form Ka = / . General Kb expressions take the form Kb = / .
Keeping this in consideration, what is KA and KB in chemistry?
Ka is the acid dissociation constant. pKa is simply the -log of this constant. Similarly, Kb is the base dissociation constant, while pKb is the -log of the constant. The acid and base dissociation constants are usually expressed in terms of moles per liter .
What is the Ka in chemistry?
The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by Ka. This equilibrium constant is a quantitative measure of the strength of an acid in a solution.
What Does Kw Mean In Chemistry
Water contains both acidic and basic molecules. Because acids and bases will always react when put together, it simply means that water will react with itself! This sounds very strange. But in reality, it actually happens. The water molecules exchange protons in a process referred to as autoionization of water. This process can be expressed in the following equation below:
;H2O+H2OH3O++OH
In the above equation, one water molecule can be seen donating a proton and, therefore, acts as a Bronsted-Lowry acid. Then, another molecule accepts the molecule and, therefore, acts as a Bronsted-Lowry base. After the reaction, two molecules are formed, Hydronium ions and hydroxide ions. This reaction takes place all the time in any quantity of water.
If you have a sample of pure water, it means that the concentration of Hydronium ions and hydroxide ions is equal. Here is a demonstration in an equation .
In pure water: =;
It is important to note that the process demonstrated in the equation above is easily reversible because water is a weak base and a weak acid. To establish the concentrations, it is important to look at the next concept of the autoionization constant.
Recommended Reading: What Is The Molecular Geometry Of Ccl4
What Is Kw Defined As
kW stands for kilowatt. A kilowatt is simply 1,000 watts, which is a measure of power. So a 1,000 watt drill needs 1,000 watts of power to make it work, and uses 1 kWh of energy in an hour. Thats why, if you leave a TV or computer on standby, it is still using power and creating a kWh cost on your energy bill.
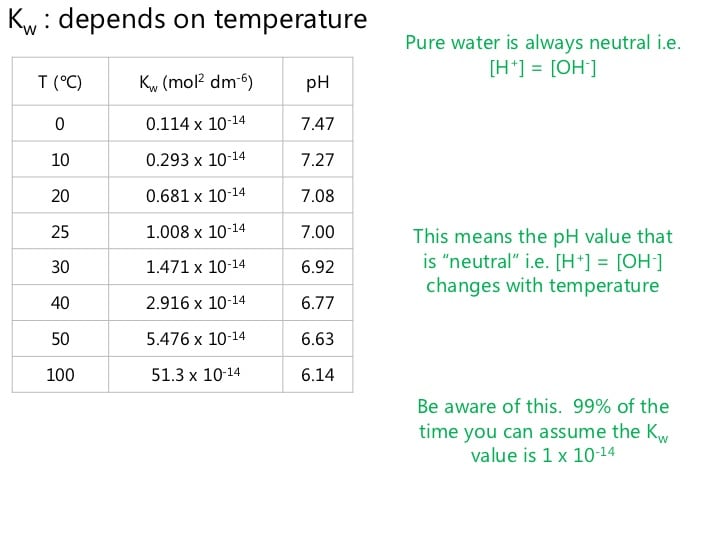
Kw Increases With Increase Of Temperature
Autoionisation of water is an endothermic process. According to Le chateliers principle, if conditions are changed in a equilibrium process, the equilibrium will shift to such a direction where it can minimize the effect of the change of the condition. Thus if water is heated the equilibrium will shift to right to form more ions by absorbing extra heat as this is an endothermic process. According to the equation of Kw, if the concentration of ions increases the;Kw increases. So we can say that Kw;increases with the increase of temperature.
Recommended Reading: Which Founding Contributors To Psychology Helped Develop Behaviorism
Introduction To The Water Ionization Constant Kw
Pure water undergoes auto-ionization or self-ionization by donating or accepting a proton between two molecules of water to form H3O+ and OH ions. This is also known as autoprotolysis or amphoteric nature of water.
The hydronium ion is a very strong acid and hydroxide ion is a very strong base. Thus they can associate again to form water molecule. So water molecules and the ions always stay in equilibrium. And the equilibrium lies to the left. Thus a very small amount of hydronium ions and hydroxide ions are found in water.
The equilibrium constant for this autoionisation of water is known as Kw. Thus
Kw = ;
Or simply;Kw = ;;;.
Here we omit the concentration of water molecule which should stay as a denominator. The reason is, not much change in concentration is observed during this process.
The Effective Range Of The Ph Scale
It is common that the pH scale is argued to range from 0-14 or perhaps 1-14 but neither is correct. The pH range does not have an upper nor lower bound, since as defined above, the pH is an indication of concentration of H+. For example, at a pH of zero the hydronium ion concentration is one molar, while at pH 14 the hydroxide ion concentration is one molar. Typically the concentrations of H+ in water in most solutions fall between a range of 1 M and 10-14 M . Hence a range of 0 to 14 provides sensible “bookends” for the scale ). One can go somewhat below zero and somewhat above 14 in water, because the concentrations of hydronium ions or hydroxide ions can exceed one molar. Figure 1 depicts the pH scale with common solutions and where they are on the scale.
Figure \: Solutions and the placement of them on pH scale
- From the range 7-14, a solution is basic. The pOH should be looked in the perspective of OH- instead lf H+. Whenever the value of pOH is greater than 7, then it is considered basic. And therefore there are more OH- than H+ in the solution
- At pH 7, the substance or solution is at neutral and means that the concentration of H+ and OH- ion is the same.
- From the range 1-7, a solution is acidic. So, whenever the value of a pH is less than 7, it is considered acidic. There are more H+ than OH- in an acidic solution.
Note
The pH scale does not have an upper nor lower bound. Negative pH values are possible
Example \
SOLUTION
| Pancreas Secretions | 8.1 |
Recommended Reading: Pre Algebra Order Of Operations Help
What Does The P Mean
Whenever you see a “p” in front of a value, like pH, pKa, and pKb, it means you’re dealing with a -log of the value following the “p”. For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.
Acidbase Properties Of Water

Recall that because of its highly polar structure, liquid water can act as either an acid or a base . For example, when a strong acid such as HCl dissolves in water, it dissociates into chloride ions ) and protons ). The proton, in turn, reacts with a water molecule to form the hydronium ion ):
In this reaction, \ is the acid, and water acts as a base by accepting an \ ion. The reaction in Equation \ref is often written in a simpler form by removing \ from each side:
In Equation \ref, the hydronium ion is represented by \, although free \ ions do not exist in liquid water as this reaction demonstrates:
Water can also act as an acid, as shown in Equation \ref. In this equilibrium reaction, \ donates a proton to \, which acts as a base:
Water is thus termed amphiprotic, meaning that it can behave as either an acid or a base, depending on the nature of the other reactant. Notice that Equation \ is an equilibrium reaction as indicated by the double arrow and hence has an equilibrium constant associated with it.
Read Also: What Is Shadowing In Psychology
Understanding Ka And Pka
Ka, pKa, Kb, and pKb are most helpful when predicting whether a species will donate or accept protons at a specific pH value. They describe the degree of ionization of an acid or base and are true indicators of acid or base strength because adding water to a solution will not change the equilibrium constant. Ka and pKa relate to acids, while Kb and pKb deal with bases. Like pH and pOH, these values also account for hydrogen ion or proton concentration or hydroxide ion concentration .
Ka and Kb are related to each other through the ion constant for water, Kw:
- Kw = Ka x Kb
Ka is the acid dissociation constant. pKa is simply the -log of this constant. Similarly, Kb is the base dissociation constant, while pKb is the -log of the constant. The acid and base dissociation constants are usually expressed in terms of moles per liter . Acids and bases dissociate according to general equations:
- HA + H2O â A- + H3O+
In the formulas, A stands for acid and B for base.
- Ka = /
- pKa = – log Ka
- at half the equivalence point, pH = pKa = -log Ka
A large Ka value indicates a strong acid because it means the acid is largely dissociated into its ions. A large Ka value also means the formation of products in the reaction is favored. A small Ka value means little of the acid dissociates, so you have a weak acid. The Ka value for most weak acids ranges from 10-2 to 10-14.
To Find The Solubility Of Solutes
Wondering how to calculate molar solubility from $K_s_p$? Knowing the value of $K_s_p$ allows you to find the solubility of different solutes. Heres an example: The $K_s_p$ value of $Ag_2SO_4$ ,silver sulfate, is 1.4×$10^^5$. Determine the molar solubility.
First, we need to write out the dissociation equation: $K_s_p$=$ ^2$ $$
Next, we plug in the $K_s_p$ value to create an algebraic expression.
1.4×$10^^5$= $^2$ $$
1.4×$10^^5$= $4x^3$
$x$==1.5x$10^^2$ M
$2x$= =3.0x$10^^2$ M
Recommended Reading: What Does F Mean In Physics
Does The Value Of Kw Remain Constant When The Solution Becomes Acidic Or Basic Due To Hydrolysis Of Salt
When few drops of acid or is added to water Kw remains constant does the same happens in case hydrolysis of salts. I want to understand the topic completely and to know about the concepts in detail
Kw represents the dissociation of water at normal condition and at 25ºC. This value is constant and is not affected whether the solution is acidic or basic. Its value is equal to 10-14. Maybe you are talking about Ka and Kb. These values represent the acid and base dissociation constants respectively which means how a certain acid is dissociated under specific conditions.
To Understand The Common Ion Effect
$K_s_p$ also is an important part of the common ion effect. The common ion effect states that when two solutions that share a common ion are mixed, the solute with the smaller $K_s_p$ value will precipitate first.
For example, say BiOCl and CuCl are added to a solution. Both contain $Cl^$ ions. BiOCls $K_s_p$ value is 1.8×$10^^31$ and CuCls $K_s_p$ value is 1.2×$10^^6$. BiOCl has the smaller $K_s_p$ value, so it will precipitate before CuCl.
You May Like: Why Biology Is The Best Science
Solving For $k_s_p$ With Solubility
In order to calculate a value for $K_s_p$, you need to have molar solubility values or be able to find them.
Question: Determine the $K_s_p$ of AgBr , given that its molar solubility is 5.71 x $10^^7$ moles per liter.
First, we need to write out the two equations.
AgBr $Ag^$ + $Br^$
$K_s_p$ =
Now, since in this problem we’re solving for an actual value of $K_s_p$, we plug in the solubility values we were given:
$K_s_p$ = = 3.26 x $10^^13$
The value of $K_s_p$ is 3.26 x $10^^13$
The Relationship Among Ph Poh And \
The pH scale is a concise way of describing the \ concentration and hence the acidity or basicity of a solution. Recall that pH and the \ ) concentration are related as follows:
\ \label\]
\=10^ \label\]
Because the scale is logarithmic, a pH difference of 1 between two solutions corresponds to a difference of a factor of 10 in their hydronium ion concentrations. Recall also that the pH of a neutral solution is 7.00 ), whereas acidic solutions have pH < 7.00 ) and basic solutions have pH > 7.00 ).
Similar notation systems are used to describe many other chemical quantities that contain a large negative exponent. For example, chemists use an analogous pOH scale to describe the hydroxide ion concentration of a solution. The pOH and \ are related as follows:
\ \label\]
\=10^ \label\]
The constant \ can also be expressed using this notation, where \.
Because a neutral solution has \, the pOH of a neutral solution is 7.00. Consequently, the sum of the pH and the pOH for a neutral solution at 25 °C is 7.00 + 7.00 = 14.00. We can show that the sum of pH and pOH is equal to 14.00 for any aqueous solution at 25 °C by taking the negative logarithm of both sides of Equation \ref:
\ &=\log \\ &= +\\ &= pH+pOH \label \end\]
For any neutral solution, pH + pOH = 14.00 with pH=pOH=7.
Example \
The Kw for water at 100 °C is \. Calculate \ for water at this temperature and the pH and the pOH for a neutral aqueous solution at 100 °C. Report pH and pOH values to two decimal places.
Given: \
Also Check: Geometry Segment Addition Postulate Worksheet