The Number Of Water Molecules
Every water molecule has a refined occupancy in the PDB file. The majority of water has an occupancy value of 1.0 indicating the presence of this water in all protein molecules forming the crystal. Some waters have occupancies of less than 1.0 indicating the water is not present in all protein molecules in the crystal. In this study, the sum of the occupancy of each water molecule was used instead of the actual number of waters.
Possible Dangers Of Gas Hydrates
Not everyone is excited by the discovery of gas hydrates. Some people think that they could be a natural hazard rather than a natural resource. Researchers are currently trying to find the most effective way to extract methane molecules from their water cages. Some people worry that as a result of the extraction methane will enter the atmosphere and affect the Earth’s climate. It’s thought that methane in the atmosphere contributes to global warming.
Gas hydrates can block natural gas pipelines and may sometimes be a drilling hazard. Another problem could result from the fact that the hydrates cement ocean sediments together. If the hydrates in a large area melt, the sediments could move. This might produce a landslide that may cause a tsunami.
How Closely Does The Hydration Water In Protein Crystals Represent Water In Solvated Proteins
Water and protein structures are affected by many factors such as temperature and ion concentration. Protein crystals are grown from solutions with various buffers, salts and/or organic precipitating agents and additives. Many protein crystals are grown at room temperature and atomic resolution X-ray data recorded at very low temperature . However, these factors were ignored in the calculations of the normalized RDF in protein crystal structures, which raises several questions as discussed below.
Crystals are often grown in high ionic strength solutions which could affect the RDF. The high ion concentration in the hydration water of protein crystals, based on previous studies , , does not increase the sharpness of RDF maxima. The RDF of 10 M lithium chloride solution has no change relative to the RDF of pure water . However, with an 8M NaCl solution the sharpness of the RDF maxima decreased about 30% from that of pure water .Salts and buffers tend to reduce the sharpness if they have any affect. The RDF in this study shows an increase in sharpness for RDF maxima , which strongly suggests that the effects of the salts are not causing the difference between hydration water and bulk water.
Read Also: Age Word Problems With 3 Variables
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Role Of Water In New Crust Formation

As aqueous fluids and hydrous melts leave the subducted slab at subarc depths, they encounter higher temperatures due to the inverted thermal gradient at the top of the slab. Water-fluxed partial melting of the mantle occurs a few kilometers above the subducted slab, when temperatures exceed about 1025 °C and melt fractions increase until the hottest part of the mantle wedge is reached . The resulting hydrous basalts to basaltic andesites ascend to form new continental crust . Compared to mid-ocean ridge basalts these arc basalts are enriched in H2O and a suite of incompatible trace elements extracted by the fluid phase from the subducted slab . Olivine-hosted melt inclusions suggest that primitive arc magmas contain about 46 wt% H2O . Water strongly affects the way the basaltic magmas differentiate. The presence of water promotes amphibole crystallization and drives plagioclase to more anorthite-rich compositions. Due to the elevated oxygen fugacity in arc magmas, magnetite forms relatively early during this differentiation. Together these three processes lead to a pronounced enrichment of SiO2 and to a differentiation producing voluminous amounts of granites . Additionally, the liberation of H2O during the crystallization of hydrous magmas and the heat input by mafic underplating promotes partial melting in the lower crust, providing an additional process for producing granites that can migrate upward to form highly differentiated upper crust .
Also Check: Define Elastic Force
Position In The Crystal Structure
A salt with associated water of crystallization is known as a hydrate. The structure of hydrates can be quite elaborate, because of the existence of hydrogen bonds that define polymeric structures.Historically, the structures of many hydrates were unknown, and the dot in the formula of a hydrate was employed to specify the composition without indicating how the water is bound. Examples:
- CuSO4· 5 H2O copper sulfate pentahydrate
- CoCl2· 6 H2O cobalt chloride hexahydrate
- SnCl2· 2 H2O tin chloride dihydrate
For many salts, the exact bonding of the water is unimportant because the water molecules are made labile upon dissolution. For example, an aqueous solution prepared from CuSO4· 5 H2O and anhydrous CuSO4 behave identically. Therefore, knowledge of the degree of hydration is important only for determining the equivalent weight: one mole of CuSO4· 5 H2O weighs more than one mole of CuSO4. In some cases, the degree of hydration can be critical to the resulting chemical properties. For example, anhydrous RhCl3 is not soluble in water and is relatively useless in organometallic chemistry whereas RhCl3· 3 H2O is versatile. Similarly, hydrated AlCl3 is a poor Lewis acid and thus inactive as a catalyst for Friedel-Crafts reactions. Samples of AlCl3 must therefore be protected from atmospheric moisture to preclude the formation of hydrates.
Epsom Salts Borax And Glauber’s Salt
Epsom salts are named after the town of Epsom in England, where they were discovered. They are used in different formulations as bath salts and as soil fertilizer. Many people claim that magnesium from the chemical can be absorbed through the skin. There is little scientific evidence for this process, however.
Borax is used as a household cleaner and is present in low concentrations in some cosmetics. There are multiple concerns about its safety. It mustnt be ingested and should be kept out of reach of children and pets.
Glaubers salt is named after Johann Rudolf Glauber, a German-Dutch chemist and apothecary who lived in the seventeenth century. Glauber discovered sodium sulphate and also discovered that it acts as a laxative in humans.
Also Check: Olaplex Vs Redken
Meaesurement Of Hydration By Radio Waves:
The technique would consist of using a device that looks like a wristwatch, thus providing a convenient and painless alternative to existing, more invasive methods.
It is normal, during intense physical activity, to lose between 2 and 4% of your total body weight, due to dehydration caused by sweating . Severe dehydration however, corresponding to an 8% loss of body weight, can be fatal.
Measuring hydration level is important for categories such as athletes, the elderly and malnourished children.
Existing methods requiring blood collection, while being accurate, may not be feasible in an emergency.
Other methods, such as measuring the bodys electromagnetic impedance, are not sufficiently precise.
Yair Shapiro and his colleagues at the Heller Institute of Medicine observed how radio waves of various frequencies were absorbed by subjects subjected to exercise sessions. To do this, they placed on the wrists of the subjects who had taken part in the experiments, a small device absorbing radiofrequencies.
They then observed a definite correlation between weight loss and absorption of radio waves. The researchers plan to conduct observations on other groups, in order to verify these results.
Read it also:
The Importance Of Hydration
- Department of Energy, Office of Science
- Summary:
- In all organisms, water’s pH has a profound effect. Because the interaction of carbon dioxide and water explains the natural acidity of water and all accompanying reactions, it is considered a vital reaction by scientists. Researchers recently made a discovery about how dissolved dioxide bonds.
In people, phytoplankton, and in fact in all living organisms, water’s pH — acidic, basic, or neutral — has a profound effect. Water often becomes acidic because of contacting gaseous carbon dioxide in the atmosphere. When the carbon dioxide dissolves in the water, it forms carbonic acid. DOE scientists characterized the structure of carbon dioxide in water. They found that the dissolved carbon dioxide bonds only very weakly to the surrounding water, but creates a cylindrical cavity in the liquid.
The interaction of carbon dioxide and water is one of the most important reactions in all of science, given that it explains the natural acidity of water and all accompanying reactions. Identifying the structure of dissolved carbon dioxide provides the starting point for all research that attempts to address this all-important reaction.
The results of this study will serve as the foundation for future research into this crucial chemical reaction.
Story Source:
Recommended Reading: Linear Span
What Is Known As Water Of Crystallization
In chemistry, water of crystallization or water of hydration are water molecules that are present inside crystals. Classically, water of crystallization refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
Why is water of crystallization important?
Role of water of crystallization is to enable the substance to form crystals. THAT is WHY to form crystals, water is allowed to evaporate slowly at normal or slightly high temperature because at very high temperature all the water would vigorously vaporize, not leaving behind any water for crystallization.
A Hydrate Quiz For Review And Fun
For each question, choose the best answer. The answer key is below.
- Aldehydes and ketones information from Michigan State University
- Information about the formation of hydrates from aldehydes and ketones from the University of Calgary
- Methane hydrate information from the U.S. Department of Energy
This content is accurate and true to the best of the authors knowledge and does not substitute for diagnosis, prognosis, treatment, prescription, and/or dietary advice from a licensed health professional. Drugs, supplements, and natural remedies may have dangerous side effects. If pregnant or nursing, consult with a qualified provider on an individual basis. Seek immediate help if you are experiencing a medical emergency.
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
Hydration Enthalpy And Solubility
The ions in a solute are bound together by coulombic force of attraction, to dissolve this solute into the solvent the water molecule should overcome this strong force of attraction. The energy required to break this string force of attraction is called lattice enthalpy.
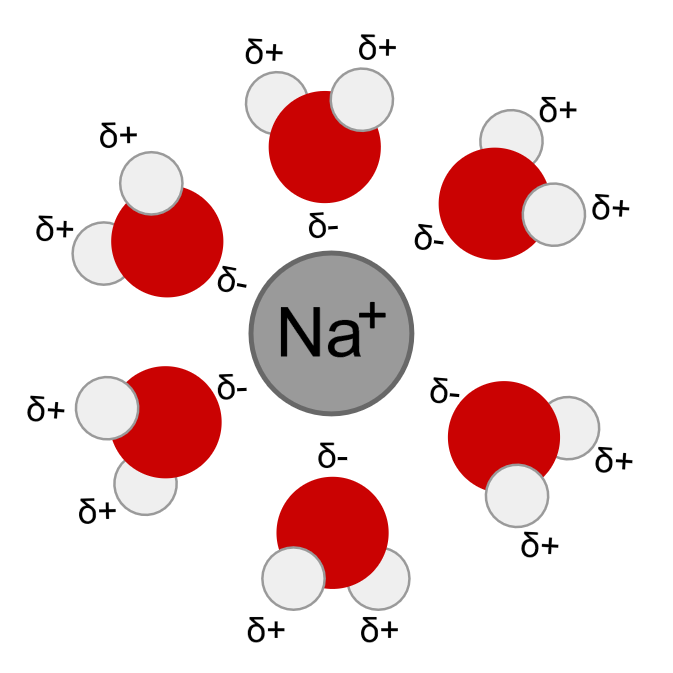
Most of the ionic compounds are insoluble in non-aqueous solutions but they show high solubility in water. The factor that determines the solubility of a salt is the interactions of the ions with the solvent. As explained earlier the water is a polar molecule with a partial positive charge on hydrogen and partial negative charge on oxygen, interacts with the ions and forms a strong bond releasing energy.
The process of dissolution can be considered as a combination of two processes.
The first one is,
M+ M+ = HLatt Lattice enthalpy
The second process is the hydration,
M+ + aq M+ = HHyd Hydration enthalpy
The first process involves the breaking of bonds in the solid solute therefore, it is an endothermic process. Lattice enthalpy can be defined as the energy released when one mole ionic solid is converted gaseous ions. Greater the lattice enthalpy greater energy is required to overcome the force of attraction. Some compounds are insoluble in water because of their higher lattice enthalpy value.
Also Read: Entropy
HHyd = HHyd + HHyd
The enthalpy of the solution involves two processes, i.e., lattice energy and enthalpy of hydration.
NaCl Na+ +Cl
Na+ + Cl Na+ + Cl
NaCl Na+ + Cl
Where,
Recycling Of Water Through Subduction Zone Metamorphism
The altered oceanic crust is buried along subduction zones, providing a mechanism for transporting water and carbon into the mantle . Aqueous fluids are then produced by dehydration reactions involving the hydrous minerals introduced in the previous section during burial and heating of the oceanic crust . Sediments play a quantitatively minor role for water storage and thus are not considered here. In the altered mafic oceanic crust, key hydrous minerals formed by progressive reequilibration of the weathering products of the oceanic crust during burial in the subduction zone include chlorite, amphibole, epidote, and lawsonite. With rising temperature and pressure, these hydrous minerals are subjected to dehydration reactions that generally occur over an extended temperature range characteristic of the forearc-to-subarc of subducting slabs . This leads to a gradual release of water that is most pronounced at fore-arc conditions, up to 80 km depth . At subarc conditions mafic rocks have mainly converted to an anhydrous rock called eclogite that dominantly consists of garnet and omphacite. In contrast, in subducted hydrated mantle, there are only three major dehydration reactions occurring over restricted temperature intervals of about 2030 °C and correspond to the release of ~2 wt% H2O , 58 wt% H2O , and 13 wt% H2O .
Read Also: Unit 1 Geometry Basics Homework 2 Segment Addition Postulate Answer Key
Hydration/dehydration In Central Metabolism
There are numerous reactions in central metabolism, the locus of chemical reactions that supply material and energy to the living system, which involve addition or elimination of water. Core carbon metabolism uses vicinal dehydrations in the interconversion of metabolic intermediates. These include the interconversion of citrate and isocitrate by aconitase and of fumarate and malate by fumarase in the TCA cycle . The TCA cycle is the major energy-yielding catabolic pathway in the cells, and its intermediates are fundamental for cellular biosynthesis. Metabolic substrates as sugars, lipids, and amino acids enter the TCA cycle as acetyl-CoA and are oxidized to CO2. The cycle starts with the condensation through a hydration reaction of the acetyl group from acetyl-CoA to oxaloacetate to form citrate, which is promptly dehydrated and re-hydrated to isocitrate thanks to the action of the aconitate hydratase. This hydration/rehydration represents a critical step for the TCA cycle since citrate, a tertiary alcohol, cannot be easily oxidized. Another critical hydration step during the TCA cycle is the reverse conversion of fumarate to L-malate catalyzed by fumarate hydratase.
Hydration Enthalpy Of Elements
Hydration enthalpy values of various elements are tabulated in the table given below.
| Ion | |
| I- | -296 |
The magnitude of hydration enthalpy depends on the charge density of the ions. The charge density is more for smaller ions and hence the smaller ions have higher values of hydration enthalpy. The higher the charge density the higher will be the force of attraction between the ion and the water polar end. This makes the value of hydration enthalpy higher in smaller ions. The alkali metals are highly hydrated, and the extent of hydration decreases down the group.
Don’t Miss: How To Solve For Half Life Chemistry
The Water Radial Distribution Of B
The water radial distribution of B-factor is the averaged B-factor of water as a function of water-water distance. At any distance from water w , the radial distribution function of water B-factor can be calculated as
Where W is the number of water and partial water molecules in the ADV with radial from water w , bw is B-factor of each water in the ADV, ow is the occupancy of each water in the ADV. Thus, the averaged RDFB over all sample crystal structures can be expressed as
Where P is the total number of protein structures, Wp is the total number of water molecules in protein p, rwp is the distance from the UDV to water w in protein p.
Gas Hydrates And Their Potential Uses
Chunks of gas hydrates look like lumps of ice and appear to be crystalline solids. The building blocks of the hydrates are made at low temperature and high pressure when water molecules surround a gas molecule, forming a frozen mesh or cage. The gas is often methane, in which case the name methane hydrate may be used for the hydrate, but it may also be carbon dioxide or another gas. The methane is produced by bacterial decay of dead plants and animals. Methane has the formula CH4.
Gas hydrates have been located around the world. They form in sediments at the bottom of deep oceans and lakes and are also found on land in permafrost. Methane hydrates have the potential to be an excellent source of energy. In fact, researchers estimate that the total amount of energy trapped in the world’s gas hydrates may be greater than the total energy present in all known fossil fuels on Earth. If a gas hydrate is lit by a match or another flame, it will burn like a candle.
Read Also: Unit Test Algebra 1
What Are The Symptoms Of Dehydration
When we talk about hydration, it is important to stress that all living organisms are made up of water . This water does not appear in liquid and visible form, but it makes up muscles, bones and various interior tissues. Thus, faced with the loss of fluid from the body, the phenomenon of dehydration occurs. She has the following symptoms :
- Cramps
- Nausea and vomiting