Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
What Is Ml Chemistry
mlml
. People also ask, what does ml quantum number mean?
Magnetic Quantum Number : ml = -l, , 0, , +l. Specifies the orientation in space of an orbital of a given energy and shape . This number divides the subshell into individual orbitals which hold the electrons there are 2l+1 orbitals in each subshell.
Furthermore, what is meant by 3p3 in chemistry? minimum amount of energy that can be gained or lost. Aufbau Principle. electrons to enters the lowest energy orbital. 3p3.
Also asked, how do you find L in chemistry?
The number of values of the orbital angular number l can also be used to identify the number of subshells in a principal electron shell:
What is quantum number in chemistry?
A quantum number is a value that is used when describing the energy levels available to atoms and molecules. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the Schrödinger wave equation for the hydrogen atom.
What Are The 4 Quantum Numbers And Their Symbols
Quantum Numbers
- To completely describe an electron in an atom, four quantum numbers are needed: energy , angular momentum , magnetic moment , and spin .
- The first quantum number describes the electron shell, or energy level, of an atom.
- The dynamics of any quantum system are described by a quantum Hamiltonian .
Ekhiotz Ramisa
| n |
|---|
subshellSubshells
Abdelhadi Villaescusa
Hund’s RuleHund’s rule
Alena
angular momentum quantum numbermagnetic quantum numberspin quantum number
Yasser Vignaud
m
milliliters1 milliliterequal to 11 milliliterequal
Amberly Agranowich
Also Check: Holt Geometry Lesson 4.5 Practice B Answers
What Is The Meaning Of Ml Abbreviation In Chemistry
What is ML definition ?
ML definition is “Methyl Laurate”.
What does ML mean in Chemistry?
ML mean that “Methyl Laurate” for Chemistry.
What is ML acronym ?
ML acronym is “milliliter 1/1000 liter”.
What is shorthand of Methyl Laurate ?
The shorthand of “Methyl Laurate” is ML.
What is the definition of ML acronym in Chemistry?
Definitions of ML shorthand is “Micro”.
What is the full form of ML abbreviation?
Full form of ML abbreviation is “milliliter 1/1000 liter”.
What is the full meaning of ML in Chemistry?
Full meaning of ML is “Micro”.
What is the explanation for ML in Chemistry?
Explanation for ML is “milliliter 1/1000 liter”.
What is the meaning of ML Abbreviation in Astrology ?
The site does not only include the meanings of the ML abbreviation in Chemistry. Yes, we know your main purpose is explanation of ML abbreviation in Chemistry. However, we thought that besides the meaning of the ML definitions in Chemistry, you can consider astrological information of ML acronym in Astrology. Therefore, the astrological explanation of each word in each ML abbreviation is also included.
ML Abbreviation in Astrology
How To Calculate Mole Fraction Of A Solution
Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. The sum of all mole fractions adds up to 1. Note that moles cancel out when calculating mole fraction, so it is a unitless value. Note some people express mole fraction as a percent . When this is done, the mole fraction is multiplied by 100%.
symbol: X or the lower-case Greek letter chi, , which is often written as a subscript
Calculate Mole Fraction: XA= /
Example: Determine the mole fraction of NaCl in a solution in which 0.10 moles of the salt is dissolved in 100 grams of water.
The moles of NaCl is provided, but you still need the number of moles of water, H2O. Start by calculating the number of moles in one gram of water, using periodic table data for hydrogen and oxygen:
- H = 1.01 g/mol
- O = 16.00 g/mol
- H2O = 2 + 16 = 18 g/mol
Use this value to convert the total number of grams of water into moles:
* 100 g = 5.56 moles of water
Now you have the information needed to calculate mole fraction.
- Xsalt= moles salt /
- Xsalt= 0.10 mol /
- Xsalt= 0.02
Also Check: Paris Jackson’s Biological Parents
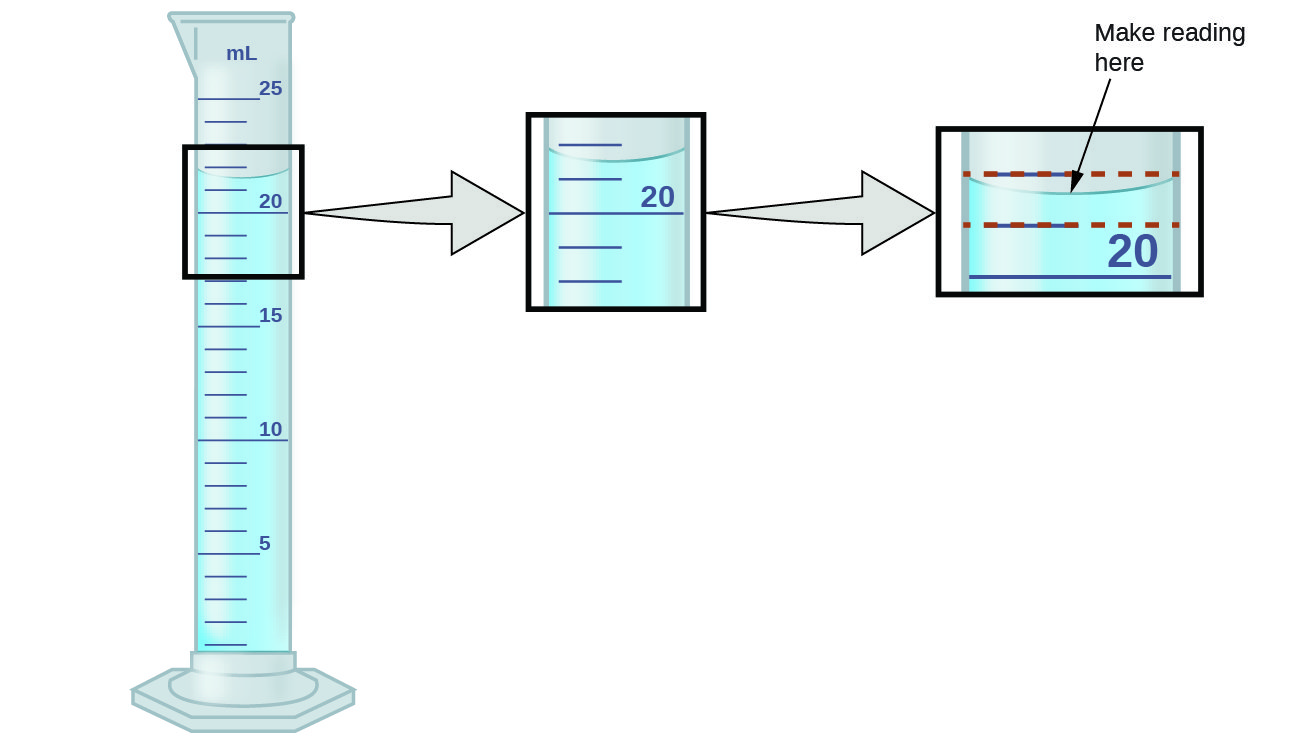
What Is A Buret Used For In Chemistry
Burette, also spelled buret, laboratory apparatus used in quantitative chemical analysis to measure the volume of a liquid or a gas. It consists of a graduated glass tube with a stopcock at one end.Apr 8, 2021
When would you use a Buret?, A buret is used to deliver solution in precisely-measured, variable volumes. Burets are used primarily for titration, to deliver one reactant until the precise end point of the reaction is reached. To fill a buret, close the stopcock at the bottom and use a funnel.
Furthermore, Why is a Buret the most accurate?, In Part A the buret is more precise in measuring the volume of a liquid than using a graduated cylinder or beaker. In part B, the buret is accurate in being able to read measurements forvolume. Using a pipet is accurate in being able to give precise volume.
Finally, What is special about a burette?, The burette delivers accurate volumes of liquid to another container, such as in a titration. When an analyst titrates two substances, he or she analyzes how much of one substance is needed to make a visually recognizable change in the other substance.
S Per Million Unit Calculations
Recall that, in general, concentration tells you how much solute is present in a solution.
concentration = amount of solute ÷ amount of solution
A concentration in parts per million may refer to the mass of solute present in the volume of solution or it may refer to the mass of solute present in a mass of solution .
In SI units, w/w concentration would be given in kilograms of solute per kilograms of solution. So, a 1 part per million solution would be 1 kg of solute per 1 million kilograms of solution. And these masses are just too large to be useful in Chemistry laboratory. But we can divide the masses of solute and solution by 1 million to arrive at more useful units:
| 1 ppm |
| 1 g solute 1 g solution |
This allows us to establish that parts per million concentration is equivalent to the following common m/m concentrations:
1 ppm = 1 mg solute/1 kg solution = 1 g solute/1 g solution
In SI units, w/v concentration would be given in kilograms of solute per litres of solution. So, a 1 part per million solution would be 1 kg of solute per 1 million litres of solution. And this mass and volume are just too large to be useful in Chemistry laboratory. But we can divide the mass of solute and volume of solution by 1 million to arrive at more useful units:
| 1 ppm |
ppm = mass of solute ÷ mass of solution
ppm = mass of solute ÷ mass of solution
You should practice rearranging the equations above in order to find mass of solute, volume of solution or mass of solution:
Don’t Miss: Why Are There Different Branches Of Chemistry
What Is The Accuracy Of A Burette
10 mL burettes are usually graduated each 0.05 mL, while 25 mL and 50 mL burettes are usually graduated each 0.1 mL. That means that 50 mL burettes have the highest resolution. 0.050 mL out of 50 mL is 0.1%, and thats about maximum precision that we can get from volume measurement when using burette.
The Electron Spin Quantum Number
Unlike \, \, and \, the electron spin quantum number \ does not depend on another quantum number. It designates the direction of the electron spin and may have a spin of +1/2, represented by, or 1/2, represented by . This means that when \ is positive the electron has an upward spin, which can be referred to as “spin up.” When it is negative, the electron has a downward spin, so it is “spin down.” The significance of the electron spin quantum number is its determination of an atom’s ability to generate a magnetic field or not. /Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electron_Spin” rel=”nofollow”> Electron Spin.)
Example \
List the possible combinations of all four quantum numbers when \, \, and \.
- Answer
-
The fourth quantum number is independent of the first three, allowing the first three quantum numbers of two electrons to be the same. Since the spin can be +1/2 or =1/2, there are two combinations:
- \, \, \, \
- \, \, \, \
Example \
-
No, if the value of \ is positive, the electron is “spin up.”
Also Check: What Is Surface Area In Math
How To Calculate Molality Of A Solution
Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same.
Calculate Molality: moles solute per kilogram solvent
symbol: m
m = moles / kilogram
Example: What is the molality of a solution of 3 grams of KCl in 250 ml of water?
First, determine how many moles are present in 3 grams of KCl. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl.
- K = 39.1 g/mol
- KCl = 39.1 + 35.5 = 74.6 g/mol
For 3 grams of KCl, the number of moles is:
* 3 grams = 3 / 74.6 = 0.040 moles
Express this as moles per kilogram solution. Now, you have 250 ml of water, which is about 250 g of water , but you also have 3 grams of solute, so the total mass of the solution is closer to 253 grams than 250. Using 2 significant figures, it’s the same thing. If you have more precise measurements, don’t forget to include the mass of solute in your calculation!
- 250 g = 0.25 kg
- m = 0.040 moles / 0.25 kg = 0.16 m KCl
What Is The Use Of Dropper In Chemistry Lab
4.5/5dropperdropperusedusedlaboratoryuse
Furthermore, what is a dropper bottle used for in science?
Glass Dropper. Glass dropper with 0.5 and 1.0 mL marks is used to measure and dispense liquids.
Similarly, what is the difference between a pipette and a dropper? Pasteur pipettes, also known as droppers are used to transfer small amounts of liquids, but are not graduated. Pasteur pipettes are made of plastic or glass. Mohr pipettes are measuring pipettes that resemble serological pipettes, with the primary difference that the graduations do not extend all the way to the tip.
Just so, what is a dropper in chemistry?
CHEMISTRY GLOSSARYDropper is a pipette consisting of a small tube with a vacuum bulb at one end for drawing liquid in and releasing it a drop at a time.
What are droppers made of?
DROPPERS. The classic dropper is made of tube glass, combined with a silicone or push-button dosing head and a drop shaped pipette. The collar can be metallized as desired or coated with an aluminum collar.
Recommended Reading: Geometry Dash All Unlocked Pc
Chemistry End Of Chapter Exercises
What is the Clâ concentration in a 0.25-mL sample of normal serum that requires 1.46 mL of 8.25 Ã 10â4M Hg2 to reach the end point?
Is A Burette Accurate Or Precise

The burette tube carries graduated marks from which the dispensed volume of the liquid can be determined. Compared to a volumetric pipette, a burette has similar precision if used to its full capacity, but as it is usually used to deliver less than its full capacity, a burette is slightly less precise than a pipette.
Recommended Reading: Define Electron Geometry
Nature Of The Particles
The mole is essentially a count of particles. Usually the particles counted are chemically identical entities, individually distinct. For example, a solution may contain a certain number of dissolved molecules that are more or less independent of each other. However, in a solid the constituent particles are fixed and bound in a lattice arrangement, yet they may be separable without losing their chemical identity. Thus the solid is composed of a certain number of moles of such particles. In yet other cases, such as diamond, where the entire crystal is essentially a single molecule, the mole is still used to express the number of atoms bound together, rather than a count of multiple molecules. Thus, common chemical conventions apply to the definition of the constituent particles of a substance, in other cases exact definitions may be specified.The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams.
The Orbital Angular Momentum Quantum Number
The orbital angular momentum quantum number \ determines the shape of an orbital, and therefore the angular distribution. The number of angular nodes is equal to the value of the angular momentum quantum number \. Each value of \ indicates a specific s, p, d, f subshell The value of \ is dependent on the principal quantum number \. Unlike \, the value of \ can be zero. It can also be a positive integer, but it cannot be larger than one less than the principal quantum number ):
Example \
If \, what are the possible values of \?
- Answer
-
Since \ can be zero or a positive integer less than ), it can have a value of 0, 1, 2, 3, 4, 5 or 6.
Example \
If \, how many angular nodes does the atom have?
- Answer
-
The number of angular nodes is equal to the value of l, so the number of nodes is also 4.
You May Like: Function Notation Common Core Algebra 1 Homework Answers
Deep Learning Deep Chemistry
In this section, an introductory overview into the core concepts of DL, and DLNs is provided. Focus is given to the unique properties of DL, that distinguish these algorithms from traditional machine learning approaches, with emphasis on chemical applications rather than providing theoretical and mathematical details.
ML is a branch of computer science dedicated to the development of algorithms capable of learning and making decisions on complex data . This learning process involves specific tasks that are commonly classified in supervised learning, for establishing the relationship between input and output data , unsupervised learning, for finding hidden patterns or features in data, without any previous information on such characteristics and interrelations , and reinforcement learning, for performing a particular task through repeated dynamic interactions e.g., optimization of molecules and chemical reactions .
Deep learning is a fast-moving sub-area of ML, focused on sophisticated learning and extrapolation tasks, fostered by the wide range of chemistry literature, open-source code, and datasets .
There are barriers to be surpassed, including cleaning data, production of meaningful and accurate chemical information , lack of standardization of chemical data, expertise and familiarity with ML and DL in chemistry sectors, and also lack of collaboration opportunities) .
The majority of DL algorithms currently developed are based on artificial neural networks .
What Does Ml Stand For
What does ML mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: ML.
Filter by:
- milliliter, millilitre, mil, ml, cubic centimeter, cubic centimetre, cc
- a metric unit of volume equal to one thousandth of a liter
Popularity rank for the ML initials by frequency of use:
Couldn’t find the full form or full meaning of ML?
Maybe you were looking for one of these abbreviations:
Discuss these ML abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
Read Also: Geometry Lesson 1.7 Answers