Nature Of The Particles
The mole is essentially a count of particles. Usually the particles counted are chemically identical entities, individually distinct. For example, a solution may contain a certain number of dissolved molecules that are more or less independent of each other. However, in a solid the constituent particles are fixed and bound in a lattice arrangement, yet they may be separable without losing their chemical identity. Thus the solid is composed of a certain number of moles of such particles. In yet other cases, such as diamond, where the entire crystal is essentially a single molecule, the mole is still used to express the number of atoms bound together, rather than a count of multiple molecules. Thus, common chemical conventions apply to the definition of the constituent particles of a substance, in other cases exact definitions may be specified.The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams.
Examples Of Values Expressed In Atomic Mass Units
- A hydrogen-1 atom has a mass of 1.007 u .
- A carbon-12 atom is defined as having a mass of 12 u.
- The largest known protein, titin, has a mass of 3 x 106 Da.
- AMU is used to differentiate between isotopes. An atom of U-235, for example, has a lower AMU than one of U-238, since they differ by the number of neutrons in the atom.
What Is The Unit Of Volume In Chemistry
unit of volumevolumevolumechemistryvolume
. Similarly, what are the units used in volume?
The basic unit of volume in the international system is the liter , although the cubic centimeter and milliliter are also widely used as units for measuring volume.
Furthermore, what is the SI unit of mass and volume? kilogram
Secondly, what is volume measured in science?
Volume, as measured in chemistry, is the amount of space that matter occupies. It is most often measured by the liter , 1.057 qt. in conventional units, or the milliliter , 1/1000 of a liter, about 0.0338 of an ounce. It is often measured by cylinders, flasks, pipettes, or syringes in and out of the laboratory.
What is volume and How Is It Measured?
Volume = length x width x height. You only need to know one side to figure out the volume of a cube. The units of measure for volume are cubic units. Volume is in three-dimensions. You can multiply the sides in any order.
Don’t Miss: Houghton Mifflin Harcourt Geometry Workbook Answers
Key Takeaways: Chemistry Unit Conversions
- Unit conversions only work if the units are the same type. For example, you can’t convert mass into temperature or volume into energy.
- In chemistry, it would be nice if you only had to convert between metric units, but there are many common units in other systems. For example, you may need to convert a Fahrenheit temperature into Celsius or a pound mass into kilograms.
- The only math skills you need to do unit conversions are addition, subtraction, multiplication, and division.
Converting Moles To Grams
One of the most common chemistry calculations is converting moles of a substance into grams. When you balance equations, you’ll use the mole ratio between reactants and reagents. To do this conversion, all you need is a periodic table or another list of atomic masses.
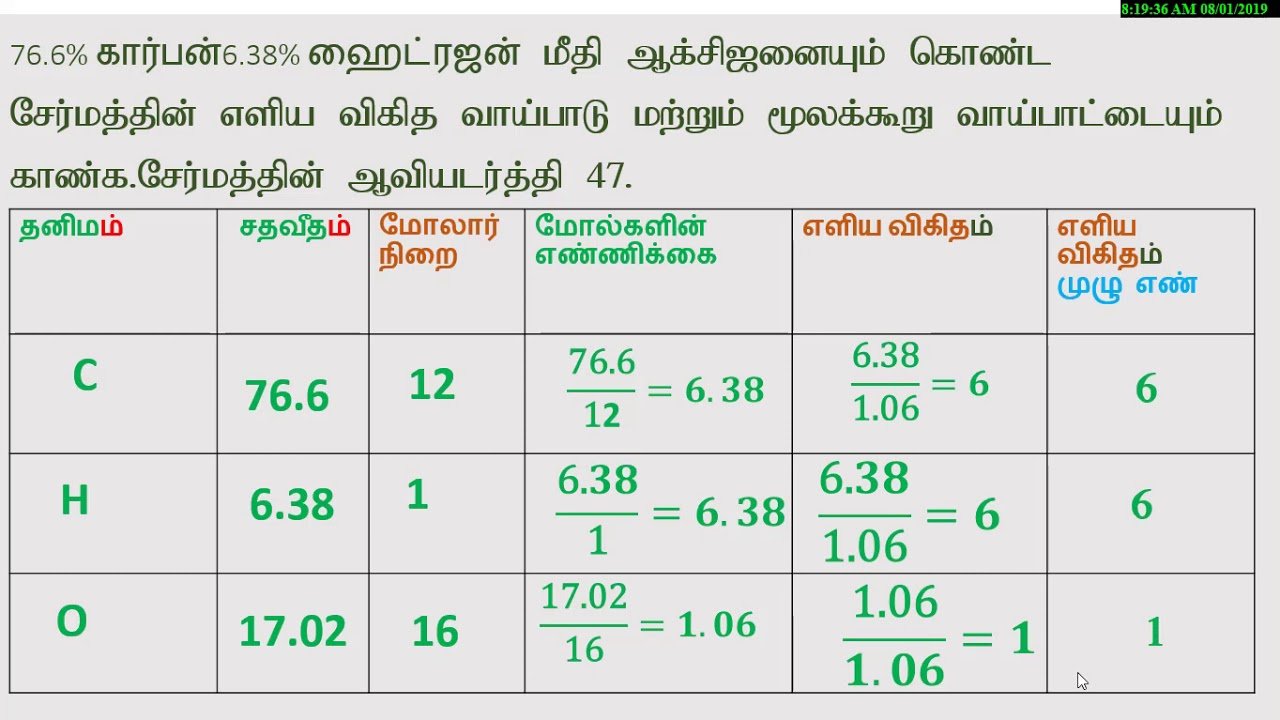
Example: How many grams of carbon dioxide is 0.2 moles of CO2?
Look up the atomic masses of carbon and oxygen. This is the number of grams per one mole of atoms.
Carbon has 12.01 grams per mole.Oxygen has 16.00 grams per mole.
One molecule of carbon dioxide contains 1 carbon atom and 2 oxygen atoms, so:
number of grams per mole CO2= 12.01 + number of grams per mole CO2= 12.01 + 32.00number of grams per mole CO2= 44.01 gram/mole
Simply multiply this number of grams per mole times the number of moles you have in order to get the final answer:
grams in 0.2 moles of CO2= 0.2 moles x 44.01 grams/molegrams in 0.2 moles of CO2= 8.80 grams
It’s good practice to make certain units cancel out to give you the one you need. In this case, the moles canceled out of the calculation, leaving you with grams.
You can also convert grams to moles.
You May Like: Spans Linear Algebra
Units Of The Si System
There are seven base units in the SI system:
- the kilogram , for mass
- the second , for time
- the kelvin , for temperature
- the ampere , for electric current
- the mole , for the amount of a substance
- the candela , for luminous intensity
- the meter , for distance
The Seven SI Units: This figure displays the fundamental SI units and the combinations that lead to more complex units of measurement.
It should be apparent that the move into modern times has greatly refined the conditions of measurement for each basic unit in the SI system, making the measurement of, for example, the luminous intensity of a light source a standard measurement in every laboratory in the world. A light source made to produce 20 cd will be the same regardless of whether it is made in the United States, in the UK, or anywhere else. The use of the SI system provides all scientists and engineers with a common language of measurement.
Types Of Temperature Scales
Temperature can be measured and represented in many different ways. The fundamental requirements of the practice involve accuracy, a standard, linearity, and reproducibility. The SI unit, chosen for its simplicity and relationship to thermodynamics, is the kelvin, named in honor of Lord Kelvin. While incrementally equal to the Celsius scale, the temperature in kelvins is a true representation of the kinetic energy in a thermodynamic sense. Chemistry and physics require many calculations involving temperature. Those calculations are always made in kelvins.
Comparison of temperature scales: Temperatures of some common events and substances in different units.
A comparison of temperature scales table illustrates a variety of temperature scales, some of which are no longer used. It is interesting to see the temperatures of commonly occurring events over these scales, and to imagine the great hurdles that were overcome in developing modern thermometry.
Conversion to and from kelvin: Use the equations in this table to calculate temperatures using the kelvin measurement system.
Although in most cases scientists are equipped with some sort of electronic calculator, there might be times when a conversion from one scale to another is required. Conversion tables can be used to convert a measurement to any scale from any other temperature scale, such as kelvin or Celsius.
Don’t Miss: Homework 4 Angle Addition Postulate Answers
Definition By The Iupac
The existence of two distinct units with the same name was confusing, and the difference was large enough to affect high-precision measurements. Moreover, it was discovered that the isotopes of oxygen had different natural abundances in water and in air. For these and other reasons, in 1961 the International Union of Pure and Applied Chemistry , which had absorbed the ICAW, adopted a new definition of the atomic mass unit for use in both physics and chemistry namely, 112 of the mass of a carbon-12 atom. This new value was intermediate between the two earlier definitions, but closer to the one used by chemists .
The new unit was named the “unified atomic mass unit” and given a new symbol “u”, to replace the old “amu” that had been used for the oxygen-based units. However, the old symbol “amu” has sometimes been used, after 1961, to refer to the new unit, particularly in lay and preparatory contexts.
With this new definition, the standard atomic weight of carbon is approximately 12.011 Da, and that of oxygen is approximately 15.999 Da. These values, generally used in chemistry, are based on averages of many samples from Earth’s crust, its atmosphere, and organic materials.
What Does The M Mean In Chemistry
. In this way, what does italicized m mean in chemistry?
Both m and M are units of the concentration of a chemical solution. The lowercase m indicates molality, which is calculated using moles of solute per kilograms of solvent. Uppercase M is molarity, which is moles of solute per liter of solution .
One may also ask, is M the same as mol L? Molar concentration. In chemistry, the most commonly used unit for molarity is the number of moles per litre, having the unit symbol mol/L. A solution with a concentration of 1 mol/L is said to be 1 molar, commonly designated as 1 M.
Hereof, what does 1.0 m mean in chemistry?
Molar can also apply to the concentration of a solute in water: a 1.0 Molar solution has 1.0 moles of solute made up to 1.0 liters of solution. This would be labeled 0.1 M HCl or 0.1 M Hydrochloric Acid. A mole is 6.02 x 10^23 atoms or molecules of a given substance.
What does 0.05m mean?
This means that a 1 M solution is 40g in 1 litre of water. A 0.05 M solution will be 40*0.05 g in a litre. This is 2g in a litre.
Also Check: Linear Algebra Span Definition
The Variable Density Of Water
Water itself is a complicated and unique molecule. Even if the pressure is consistent, waters density will change based on the temperature. Recall that the three basic forms of matter are solid, liquid and gas . As a rule of thumb, almost all materials are more dense in their solid or crystalline form than in their liquid form place the solid form of almost any material on the surface of its liquid form, and it will sink. Water, on the other hand, does something very special: ice floats on liquid water.
Look carefully at the relationship between waters temperature and its density. Beginning at 100 °C, the density of water steadily increases, as far as 4 °C. At that point, the density trend reverses. At 0 °C, water freezes to ice and floats.
This table lists the densities of water at different temperatures and constant pressure.
| The density of water at constant pressure |
|---|
| Temp |
| 983.854 |
| The values below 0ºC refer to super cooled water |
Mole A Unit Of Measurement
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A mole is simply a unit of measurement. In fact, it’s one of the seven base units in the International System of Units . Units are invented when existing units are inadequate. Chemical reactions often take place at levels where using grams wouldn’t make sense, yet using absolute numbers of atoms/molecules/ions would be confusing, too. So, scientists invented the mole to bridge the gap between very small and very large numbers.
Here is a look at what a mole is, why we use moles, and how to convert between moles and grams.
Recommended Reading: Geometry Wars 3 Achievements
Redefinition Of The Si Base Units
The definition of the dalton was not affected by the 2019 redefinition of SI base units, that is, 1 Da in the SI is still 112 of the mass of a carbon-12 atom, a quantity that must be determined experimentally in terms of SI units. However, the definition of a mole was changed to be the amount of substance consisting of exactly 6.02214076×1023 entities and the definition of the kilogram was changed as well. As a consequence, the molar mass constant is no longer exactly 1 g/mol, meaning that the number of grams in the mass of one mole of any substance is no longer exactly equal to the number of daltons in its average molecular mass.
Primitive Cubic Unit Cell
In the primitive cubic unit cell, the atoms are present only at the corners. Every atom at the corner is shared among 8 adjacent unit cells. There are 4 unit cells in the same layer and 4 in the upper layer. Therefore, a particular unit cell has the only 1/8th of an atom. Each small sphere in the following figure represents the centre of a particle that occupies that particular position and not its size. This structure is known as an open structure.
Below is an open structure
In each cubic unit cell, there are 8 atoms at the corners. Therefore, the total number of atoms in one unit cell is
8 × 1/8 = 1 atom.
Read Also: Abiotic Biology Definition
Volume Of Hcp Unit Cell
A unit cell is the smallest representation of an entire crystal. The hexagonal closest packed has a coordination number of 12 and contains 6 atoms per unit cell. The face-centered cubic has a coordination number of 12 and contains 4 atoms per unit cell.
Volume = area of base × height
Height of unit cell = \
Area of base = \
Volume = \
Volume = \
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Don’t Miss: Geometry Book Mcdougal Littell Answers
What Is A Unit In Science
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A unit is any standard used for making comparisons in measurements. Unit conversions allow for measurements of a property that have been recorded using different unitsfor instance, centimeters to inches.
Section : Chemistry And Matter
What is Chemistry?
Everything around us is made up of chemicals. From the color that makes a rose so red to the gasoline that fills our cars and the silicon chips that power our computers and cell phonesChemistry is everywhere! Understanding how chemical molecules form and interact to create complex structures enables us to harness the power of chemistry and use it, just like a toolbox, to create many of the modern advances that we see today. This includes advances in medicine, communication, transportation, building infrastructure, food science and agriculture, and nearly every other technical field that you can imagine.
Chemistry is one branch of science. Science is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. Because the physical universe is so vast, there are many different branches of science . Thus, chemistry is the study of matter, biology is the study of living things, and geology is the study of rocks and the earth. Mathematics is the language of science, and we will use it to communicate some of the ideas of chemistry.
Figure 1.1: The Relationships Between Some of the Major Branches of Science.Chemistry lies more or less in the middle, which emphasizes its importance to many branches of science.
Physical vs. Chemical Properties
Don’t Miss: Why Was The Pail Pale Math Worksheet
Number Of Atoms In Bcc Cell
a) 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atomb) 6 face-centered atoms × 1/2 atom per unit cell = 3 atoms
Hence, the total number of atoms in a unit cell = 4 atoms
Thus, in a face-centred cubic unit cell, we have:
- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 6 face-centred atoms × 1/2 atom per unit cell = 3 atoms
Therefore, the total number of atoms in a unit cell = 4 atoms.
The Units Ppm Ppb And Ppt
Dimensionless quantities, which are also sometimes called quantities of dimension one, are generally defined as the ratio of two quantities of the same kind. Examples are refractive index n and mole fraction xi more properly called amount of substance fraction . Thus the value of a dimensionless quantity is simply a number, and it would seem that no unit is required. The International System of units, the SI, includes no units for dimensionless quantities as it is at present defined.
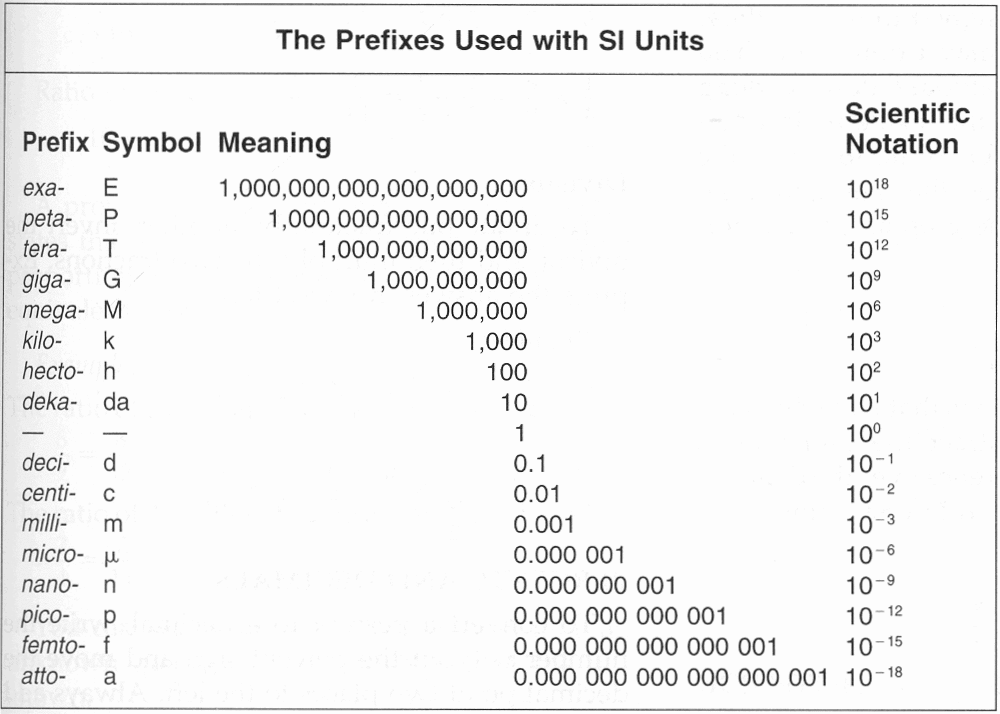
However important situations arise where the value of a dimensionless quantity is a number, which is very small compared to one, small by many powers of ten, or sometimes very large compared to one. For example the mole fraction of a minor constituent in a mixture may be of the order 106 or 109 or even smaller. When there is a unit in the value of a quantity it is customary and convenient to make use of the SI prefixes, so that instead of writing a small mass as, for example, 2.6×106 g or 2.6×109 g, we would write 2.6 µg or 2.6 ng, using the SI prefixes micro- or nano- for 106 or 109. The convenience of the SI prefixes avoids the need to use powers of ten with large negative exponents, which are clumsy to say and to type. However they are not available for dimensionless quantities, because there is no unit to which they may be prefixed.
Also Check: Is Physics Harder Than Math
What Is The Basic Unit Of Life
What distinguishes a living organism from a non-living object? Well, the answer is a living being will have self-sustaining biological processes. A cell is the smallest and the most basic form in which life exists on earth. The new cells made in the living organism came into existence from the division of the preexisting cells into two.