What Are Chemical Reactions
Chemical reactions can be described as a process in which one or more substances called reactants are converted into one or more substances known as the products. The substances, here, are either compounds or chemical elements. In simple words, when there is a rearrangement in constituent atoms of the reactants to create different substances as products, it can be said that a chemical reaction has occurred.
These reactions are an important and integral part of technology and life on Earth. Some of the common examples of chemical reactions that we witness every day are burning fuels, smelting of iron, making glass and pottery, brewing beer, and making wine and cheese.
How To Write A Net Ionic Equation
There are three steps to writing a net ionic equation:
What Is The Equilibrium Equation In Chemistry Is It Different From The Regular Equation
They are the same thing.
Explanation:
We call the short-hand notation for a chemical reaction an equation because it does involve balancing both sides and it is thus related to the mathematical equation. HOWEVER, it is not an equation in the mathematical sense, but a description of the equilibrium conditions of a chemical reaction.
In many cases the reaction proceeds primarily to the products, but even in complete reactions there will remain residual amounts of the reactants due to the equilibrium requirements of chemical interactions.
Equilibrium constants, including pH and solubilities are measures of the degree of completion or shift of a reaction from one side of the equation to the other.
ALL chemical “equations” are reversible, although the energy requirements may be excessive and make the actual event practically impossible.
Also Check: How To Figure Out Displacement
What Does Chemical Formula Mean
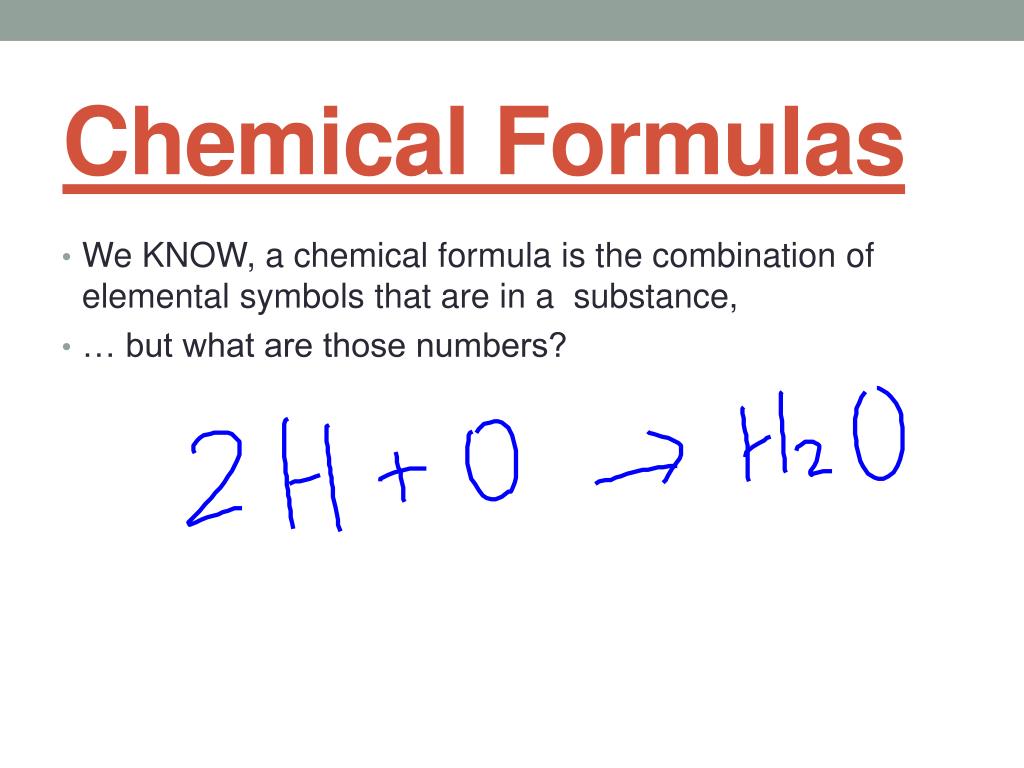
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus and minus signs.
What Is A Formula Equation
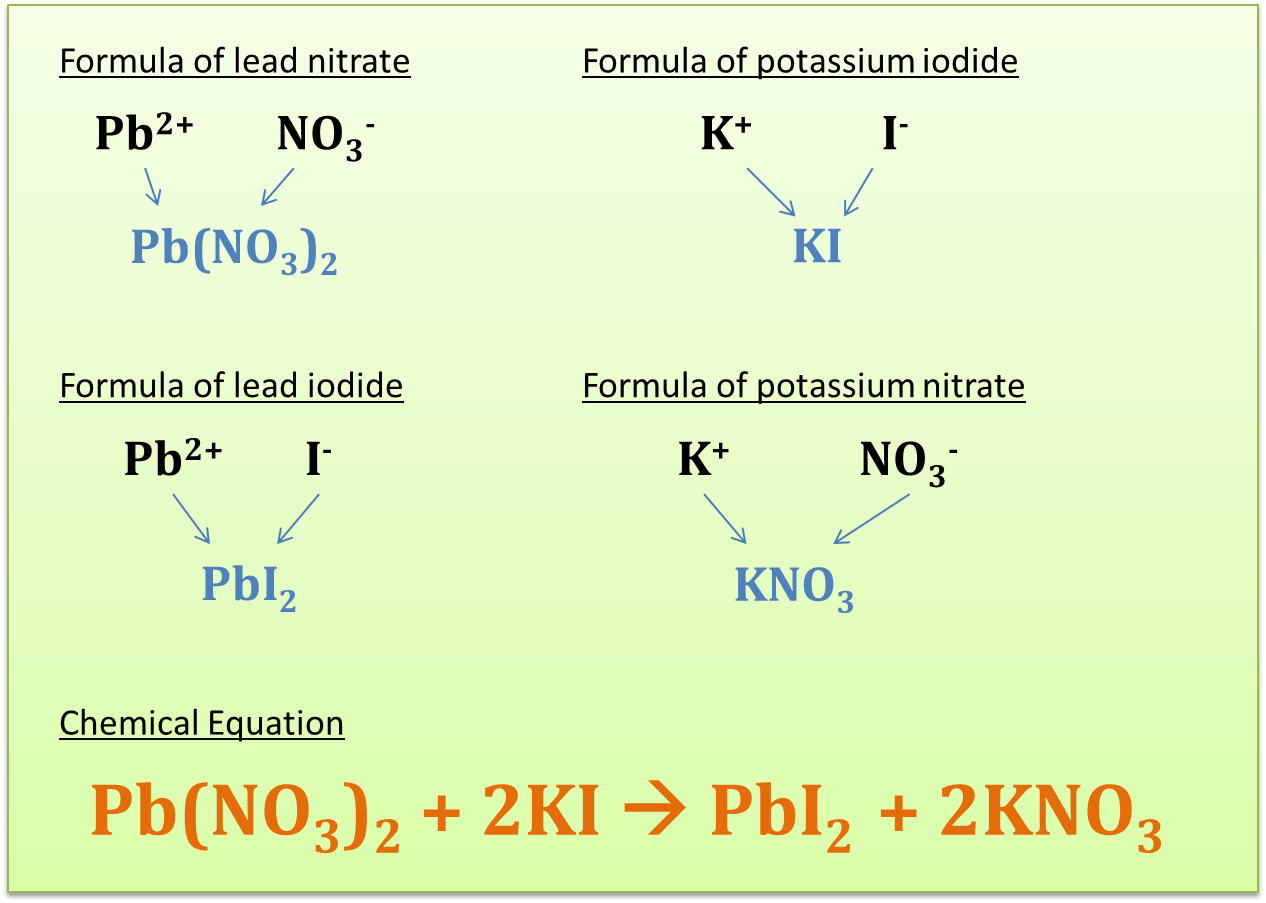
A formula equation is a visual representation of a reaction using chemical formulas. A chemical formula is an expression that states the number and types of atoms that make up any given molecule using the symbols for the elements and sub-scripted numbers.
The most well-known example of a chemical formula is water, which is H?O. Formula equations are used to keep larger and more complex equations and reactions as simple and short as possible. Furthermore, chemical formulas are standardized, so no matter where they are used, scientists know what they mean in any language. In a chemical formula equation, the reactants are listed on the left side of the equation and the result on the right.
You May Like: Algebra Nation Independent Practice Answer Key
Unbalanced And Balanced Chemical Equations
Chemical equations are either unbalanced or balanced.
- An unbalanced chemical equation lists the reactants and products and the direction the reaction proceeds, but does not specify a mole ratio between reactants and products.Example: H2 + O2 H2O
- A balanced chemical equation includes coefficients before chemical formulas and indicates the stoichiometric ratio between reactants and products. A balanced chemical equation contains equal numbers and types of atoms on both sides of the reaction arrow. It is balanced for both mass and charge.Example: 2H2 + O2 2H2O
What Is Photosynthesis For 5th Graders
Photosynthesis is the big name for the process by which plants convert energy from sunlight into energy for food. This process also requires water and carbon dioxide. In this form the plants can use the glucose,and water for food and release the oxygen back into the air for consumption by animals, including humans.
You May Like: Books Never Written Pre Algebra With Pizzazz
Importance Of Chemical Reactions
The existence of living organisms is possible on Earth because of the chemical reactions. Here are a few reasons the existence of chemical reactions is important to us:
-
Chemical reactions are required to understand the properties of the matter. These reactions help us to learn and understand the chemical properties when a sample interacts with a matter. These chemical properties can lead to the discovery of unknown specimens and explain how different types of matter react with each other.
-
The most important and beneficial discovery made by humans fire is also a product of simple chemical reactions.
-
To study outer space and other planets scientists take the help of chemical reactions. Owing to these chemical reactions we can even determine the sustainability of other planets and moons.
The direction of Chemical Reaction
It is a convention that the arrow between the reactants and products should point from left to right. However, the following symbols are used as needed in an equation:
-
For a net forward reaction, the symbol is used.
-
For a state of chemical equilibrium, the symbol is used.
-
To denote stoichiometric relationships, the = symbol is used.
-
For a reaction that occurs in both forward and backward directions, the symbol is used.
When multiple entities are used in a chemical equation, they are separated from each other with the symbol +.
Notation For A Chemical Equation
A chemical equation consists of the chemical formulas of the reactants and the products . The two are separated by an arrow symbol . Each individual substances chemical formula is separated from others by a plus sign. The state of matter of each compound or molecule is indicated in subscript next to the compound by an abbreviation in parentheses. For example, a compound in the gas state would be indicated by , solid , liquid , and aqueous . Aqueous means dissolved in water it is a common state of matter for acids, bases, and dissolved ionic compounds.
As an example, the formula for the burning of methane can be written as follows:
CH_ + 2\:O_ \rightarrow CO_ + 2\:H_O_
This equation would be read as CH four plus two O two yields CO two and two H two O. For equations involving complex chemicals, read the chemical formulas using IUPAC nomenclature, rather than reading the letter and its subscript. Using IUPAC nomenclature, this equation would be read as methane plus oxygen yields carbon dioxide and water.
You May Like: Which Pioneer In Psychology Helped Develop The School Of Thought Called Structuralism
What Is A Chemical Equation Definition And Examples
A chemical equation is a symbolic representation of a chemical reaction, indicating the reactants and products in a reaction and the direction in which the reaction proceeds. French chemist Jean Beguin gets credit for formulating the first chemical equation in 1615.
Here is an explanation of the types of chemical equations, the parts they contain, and examples.
Why Are Some Chemical Equations Unbalanced
This unbalanced chemical equation shows what substances are the reactants and what substances are the products. However, in order for this chemical equation to be of any use to us, we need to balance it, i.e. to ensure that all the atoms that are present on the reactants’ side are also present on the products’ side.
You May Like: How Many Physics Questions Are On The Mcat
Things That Should Be Considered While Planning The Pathway Of Synthesis
- Go with the reaction route, which alters only one part of the compound at one time. It reduces the formation of multiple intermediates.
- Since many reactions are occurring side by side during the synthesis reaction, they can compete. Resultantly, many side products can be formed, which lowers the yields of the desired product. Thus, go with the reaction route, which yields the product in large amounts.
- Formation of side products can be useful and problematic depending upon the nature of the by-product. A side product is only useful if it is commercially valuable. On the other hand, if it is of no value and adds to the cost of separation and purification, try to change the route to suppress its formation.
Related: Learn more about the working of spectrophotometer and capillary electrophoresis from our blogs.
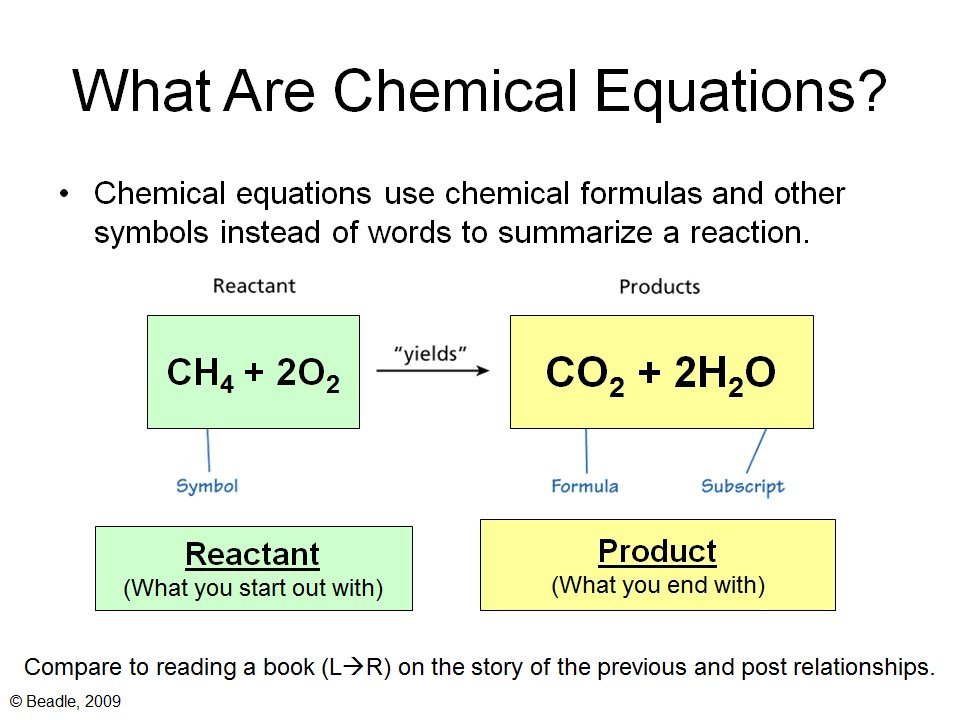
What Is A Chemical Reaction In A Chemical Equation What Does It Show Describe Each
A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compoundthe reactantson the left and the final compoundthe productson the right, separated by an arrow.
You May Like: Holt Mcdougal Geometry Workbook Answer Key
What Is A Chemical Equation
Chemical equations are symbolic representations of chemical reactions in which the reactants and the products are expressed in terms of their respective chemical formulae. They also make use of symbols to represent factors such as the direction of the reaction and the physical states of the reacting entities. Chemical equations were first formulated by the French chemist Jean Beguin in the year 1615.
Chemical reactions can be represented on paper with the help of chemical equations, an example for which is represented below .
2H2 + O2 2H2O
It can be observed in the example provided above that the reacting entities are written on the left-hand side whereas the products that are formed from the chemical reactions are written on the right-hand side of the chemical equation.
It can also be observed that there are coefficients assigned to each of the symbols of the corresponding reactants and products. These coefficients of entities in a chemical equation are the exact value of the stoichiometric number for that entity.
Additional Information In Chemical Equations
The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water . These notations are illustrated in the example equation here:
This equation represents the reaction that takes place when sodium metal is placed in water. The solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide .
Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equations arrow. For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta over the arrow.
Other examples of these special conditions will be encountered in more depth in later chapters.
Recommended Reading: What Happened To Jonathan Thomas Child Of Rage
What Is Photosynthesis For Kids
Photosynthesis is the process in which green plants use sunlight to make their own food. Photosynthesis requires sunlight, chlorophyll, water, and carbon dioxide gas. Chlorophyll is a substance in all green plants, especially in the leaves. Plants take in water from the soil and carbon dioxide from the air.
Is A Skeleton Equation Balanced
A skeleton equation doesnt show the relative and balanced amounts of reactants and products. A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. This skeleton equation shows that magnesium reacts with oxygen to form magnesium oxide.
Recommended Reading: Punchline Bridge To Algebra 2nd Ed
Chemistry End Of Chapter Exercises
What is the difference between these types of equations?
In what circumstance would the complete and net ionic equations for a reaction be identical?
\text_5 + \text_2 \text \longrightarrow \text_3 + \text
\text + \text_3 \longrightarrow \text_3)_2 + \text_2 \text + \text
\text_2 + \text_2 \longrightarrow \text
\text + \text_2 \longrightarrow \text_2 \text_3
\text + \text_2 \text \longrightarrow \text + \text_2
\text_4)_2 \text_2\text_7 \longrightarrow \text_2\text_3 + \text_2 + \text_2 \text
\text_4 + \text_2 \longrightarrow \text_3
\text_4 \longrightarrow \text + \text_2
\text + \text_2 \text + \text_2 \longrightarrow \text_2 \text + \text_2 \text
\text_4 + \text_2 \longrightarrow \text_4 \text_
\text + \text_2 \text + \text_2 \longrightarrow \text_2
\text + \text_2 \text \longrightarrow \text_3 \text_4 + \text_2
\text_2 \text_3 + \text_3 \longrightarrow \text_2 \text_4)_3
\text_3 \text_4)_2 + \text_3 \text_4 \longrightarrow \text_2 \text_4)_2
\text + \text_2 \text_4 \longrightarrow \text_2 \text_4)_3 + \text_2
\text_4 + \text_2 \text \longrightarrow \text_2 + \text
Types Of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
- Synthesis reaction
1. Combustion Reaction
A combustion reaction is a reaction with a combustible material with an oxidizer to give an oxidized product. An oxidizer is a chemical a fuel requires to burn, generally oxygen. Consider the example of combustion of magnesium metal.
Here, 2 magnesium atoms react with a molecule of oxygen producing 2 molecules of the compound magnesium oxide releasing some heat in the process.
2. Decomposition Reaction
A Decomposition reaction is a reaction in which a single component breaks down into multiple products. Certain changes in energy in the environment have to be made like heat, light or electricity breaking bonds of the compound. Consider the example of the decomposition of calcium carbonate giving out CaO which is a major component of cement.
\ \overset Ca O + CO_2 \)
Here, the compound Calcium carbonate when heated breaks down into Calcium Oxide and Carbon Dioxide.
3. Neutralization Reaction
Here, an acid and a base, Hydrochloric acid and Sodium Hydroxide react in a neutralization reaction to produce Sodium Chloride and water as the products.
4. Redox Reaction
A REDuction-OXidation reaction is a reaction in which there is a transfer of electrons between chemical species. Let us consider the example of an electrochemical cell-like redox reaction between Zinc and Hydrogen.
Don’t Miss: Bridge To Algebra Pizzazz
Dalton’s Law Of Definite Proportions
Dalton’s law of definite proportions holds true for all chemical reactions . In essence, this law states that a chemical reaction always proceeds according to the ratio defined by the balanced chemical equation. Thus, you can interpret the balanced methane equation above as reading, “one part methane reacts with two parts oxygen to produce one part carbon dioxide and two parts water.” This ratio always remains the same. For example, if we start with two parts methane, then we will consume four parts O2 and generate two parts CO2 and four parts H2O. If we start with excess of any of the reactants , the excess reactant will not be consumed:
| C | 3O2 |
| Excess reactants will not be consumed. |
In the example seen above, 3O2 had to be added to the right side of the equation to balance it and show that the excess oxygen is not consumed during the reaction. In this example, methane is called thelimiting reactant.
Comprehension Checkpoint
Dalton’s law of definite proportions states that in chemical reactions,
Constructing Balanced Chemical Equations
Aim: To construct balanced chemical equations.Materials: Copper carbonate powder, limewater, concentrated hydrochloric acid, concentrated ammonia solution, lead nitrate solution and potassium iodide solution.Apparatus: Test tubes, stoppers, rubber bung with delivery tube, test tube holder, Bunsen burner and glass tube.Procedure:A. Heating of copper carbonate
B. Formation of ammonium chloride
C. Precipitation of lead iodide
Observations:
| A yellow precipitate is produced. | The yellow precipitate is lead iodide. |
Discussion:
Also Check: Is Physics Harder Than Chemistry
Eliminating The A Factor From The Arrhenius Equation
Considering a chemical reaction at two different temperatures T1 and T2, whose corresponding rate constants are k1 and k2 respectively, the logarithmic form of the Arrhenius equation is:
ln k1 = ln Ea/RT1
The second equation can be rearranged to get the value of ln:
ln = ln + Ea/RT2
Substituting the value of ln in the equation for ln, the following equations can be obtained:
ln = ln + Ea/RT2 Ea/RT1
Shifting ln to the LHS, the value of ln ln becomes:
ln ln = Ea/RT2 Ea/RT1
The LHS of the equation is of the form ln ln, which can be simplified to ln. Also, the term Ea/R is a common factor to both the terms in the RHS. Therefore, the entire equation can be simplified as follows:\ = \frac}\)
Example For Chemical Equation
Photosynthesis is the process in plants and certain other organisms that uses the energy from the sun to convert carbon dioxide and water into glucose and oxygen.
Chemical Equation for Photosynthesis
6CO2 + 6 H2O C6H12O6 + 6O2
Where:C6H12O6 = glucoseO2 = oxygen
The above chemical equation for photosynthesis describes how the chemical reaction of carbon dioxide, water, and light energy produces carbohydrates and oxygen.
Coefficients assigned to each of the symbols indicate the stoichiometric numbers whereas subscripts indicate the number of atoms of an element present in a chemical species.
You May Like: Geometry Dash Hack No Survey