Protecting Groups Are Like Painters Tape
Its reminiscent of a problem anyone who has painted a room would understand. Imagine youre helping your cousin paint his room in a hideous shade of yellow-green so completely uncool to the untrained eye that only a hipster could appreciate it. Then you come to one of those annoying wall outlets. You could paint over it of course.
But now its useless if your cousin wants to plug in that 1965 Smith-Corona electric typewriter he found at a thrift store that hes using to write his novel. Surely theres a way to do this that doesnt destroy our outlet. So what do you do?
Painters tape to the rescue!
Cover the outlet with painters tape, paint to your hearts content, then remove the tape. THEN you can plug in the typewriter. Simple!
Reactivity Of Benzylic Centers
The enhanced reactivity of benzylic positions is attributed to the low bond dissociation energy for benzylic CH bonds. Specifically, the bond C6H5CH2H is about 1015% weaker than other kinds of CH bonds. The neighboring aromatic ring stabilizes benzyl radicals. The data tabulated below compare benzylic CH bond to related CH bond strengths.
| Bond | |
|---|---|
| akin to allylic CH bondssuch bonds show enhanced reactivity | |
| H3CH | one of the strongest aliphatic CH bonds |
| C2H5H | slightly weaker than H3CH |
| C6H5H | comparable to vinyl radical, rare |
| CH2=CHCH2H | |
| more activated vs diphenylmethyle | |
| 2CHH | |
| 339 | “triply benzylic” |
The weakness of the CH bond reflects the stability of the benzylic radical. For related reasons, benzylic substituents exhibit enhanced reactivity, as in oxidation, free radical halogenation, or hydrogenolysis. As a practical example, in the presence of suitable catalysts, p–xylene oxidizes exclusively at the benzylic positions to give terephthalic acid:
- CH3C6H4CH3 + 3 O2 HO2CC6H4CO2H + 2 H2O.
Millions of tonnes of terephthalic acid are produced annually by this method.
One Potential Solution: Ethers
As weve discussed earlier, ethers are quite possibly the most boring functional group you can encounter. The only important reaction of ethers you cover in Org 1 is how to cleave them with very strong acid . Thats it. Other than that, ethers are inert to pretty much any other reaction condition you can name.
For the chemical equivalent of painters tape, boring is good! It means that we can protect a hydroxyl group as an ether without worrying about it being affected by reactions we might like to do on the rest of the molecule .
Theres just one problem: ethers require very harsh conditions in order to break . Thats like destroying the village in order to save it: such conditions will likely torch whatever other functional groups are on your molecule.
Read Also: Algebra 1 Chapter 4 Practice Workbook Answers
What Is The Purpose Of A Protecting Group In Organic Chemistry
Protecting groups are used in synthesis to temporarily mask the characteristic chemistry of a functional group because it interferes with another reaction. A good protecting group should be easy to put on, easy to remove and in high yielding reactions, and inert to the conditions of the reaction required.
Carboxylic Acid Protecting Groups
- Methylesters Removed by acid or base.
- Benzyl esters Removed by hydrogenolysis.
- tert-Butyl esters Removed by acid, base and some reductants.
- Esters of 2,6-disubstituted phenols Removed at room temperature by DBU-catalyzed methanolysis under high-pressure conditions.
- Silyl esters Removed by acid, base and organometallic reagents.
- Orthoesters Removed by mild aqueous acid to form ester, which is removed according to ester properties.
- Oxazoline Removed by strong hot acid or alkali , but not e.g. LiAlH4, organolithium reagents or Grignard reagents
Read Also: Geometry Segment Addition Postulate Worksheet
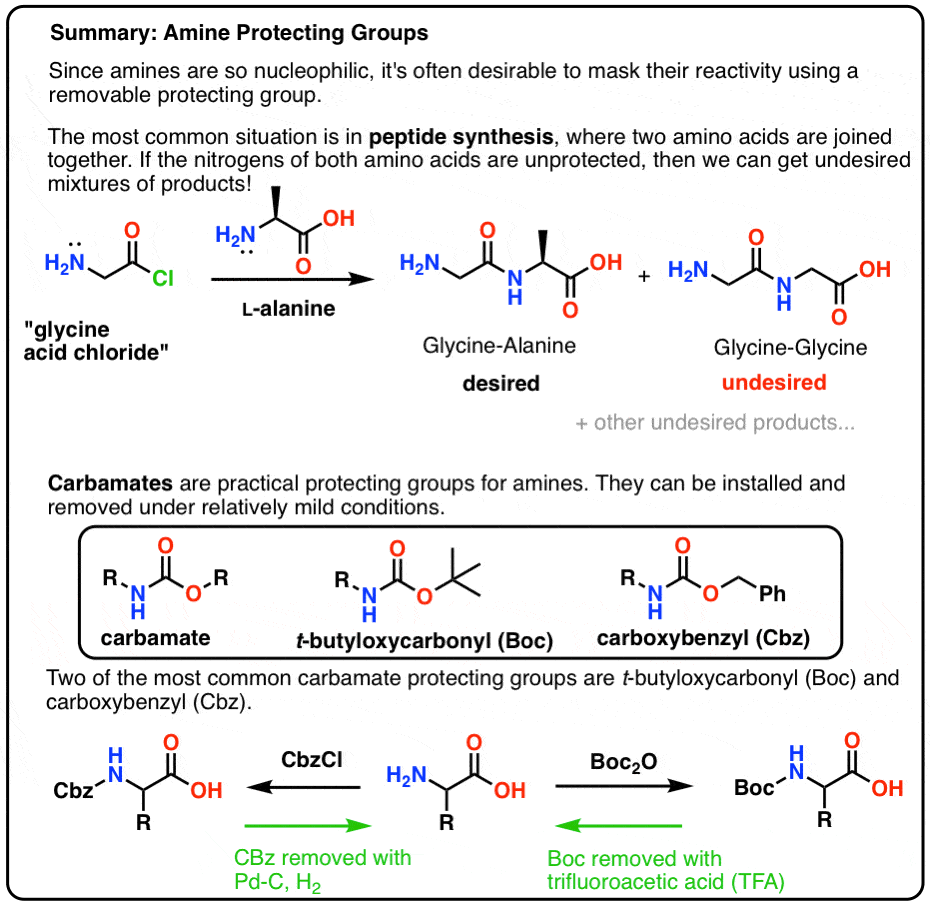
Common Amine Protection Methods
- Simple rapid stirring of a mixture of the amine and di-tert-butyl dicarbonate suspended in water at ambient temperature, an example of an on-water reaction.
- Heating a mixture of the amine to be protected and di-tert-butyl dicarbonate in tetrahydrofuran at 40 °C
- Add the amine to sodium hydroxide and di-tert-butyl dicarbonate in water and THF at 0 °C then warm to ambient temperature.
- Heating a mixture of the amine to be protected and di-tert-butyl dicarbonate in a biphasic mixture of chloroform and aqueous sodium bicarbonate at reflux for 90 minutes.
- Add the amine to di-tert-butyl dicarbonate, 4-dimethylaminopyridine , and acetonitrile at ambient temperature
BOC-protected amines are prepared using the reagent di-tert-butyl-iminodicarboxylate. Upon deprotonation, this reagent affords a doubly BOC-protected source of NHâ2, which can be N-alkylated. The approach is complementary to the Gabriel synthesis of amines.
Amino Protecting Groups Stability
| 9-Fluorenylmethyl carbamate, FMOC amino, FMOC amine, FMOC amide | |
| H2O: | |
| I2, Br2, Cl2 | MnO2/CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 503-507, 736-739.
| t-Butyl carbamate, BOC amine, BOC amino, BOC amide | |
| H2O: | |
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 518-525, 736-739.
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 531-537, 736-739.
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 550-555, 740-743.
| Trifluoroacetamide | |
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 556-558, 740-743.
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 564-566, 740-743.
| Bn-NR2 | |
| I2,Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 579-580, 744-747.
| Tr-NR2 | |
| I2,Br2, Cl2 | MnO2 / CH2Cl2 |
T. W. Green, P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 1999, 583-584, 744-747.
| Benzylideneamine | |
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
| I2, Br2, Cl2 | MnO2 / CH2Cl2 |
You May Like: Eoc Fsa Warm Ups Answers
A Chemical Equivalent Of Painters Tape
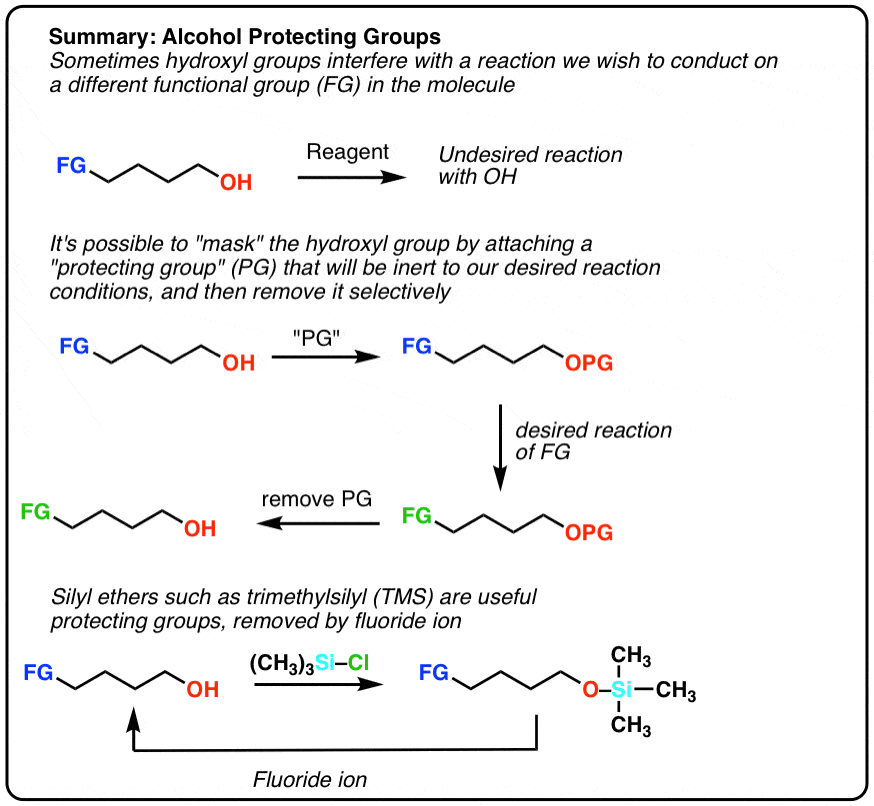
Wouldnt it be nice if we had a chemical equivalent of painters tape for alcohols. Something that could
That would allow us to perform a synthesis of our desired molecule . Here Im using PG to stand for protective group.
Well, you might have guessed by now that enterprising chemists have developed a solution for this problem. Its very clever, in fact.
When Alcohols Get In The Way
As weve seen in previous posts in this series, alcohols are very versatile functional groups that participate in a variety of reactions. They can be deprotonated with base , protonated , oxidized to aldehydes or ketones, or transformed into better leaving groups allowing for a host of substitution and elimination reactions.
All this this versatility comes with a drawback, however. Sometimes alcohol functional groups can get in the way of other reactions we might like to do. Let me show you what I mean.
Weve seen by now one of the most useful C-C bond forming reactions you learn in Org 1: nucleophilic substitution of alkyl halides with acetylides
Since alkynes are like a blank canvas, this reaction can set up the introduction of many different types of functional groups through addition reactions.
Now lets modify our substrate a bit. Well attach a hydroxyl group to the end of the molecule. Now lets see what happens.
Look at what happened we now formed a new O-CH3 bond instead of a C-C bond. What gives?
The answer, of course, is that our strong base NaNH2 deprotonated the strongest acid and the resulting alkoxide then attacked CH3-I, resulting in a substitution reaction with displacement of iodide ion
Heres another example of the the principle at work. Here, wed like to perform a substitution reaction of C-Br with C-C . So why does this reaction not lead to formation of a C-C bond?
So how could we have prevented this from occurring?
You May Like: Geometry Chapter 4 Practice Workbook Answers
Functionalization At The Benzylic Position
In a few cases, these benzylic transformations occur under conditions suitable for lab synthesis. The Wohl-Ziegler reaction will brominate a benzylic CH bond: . Any non-tertiary benzylic alkyl group will be oxidized to a carboxyl group by aqueous potassium permanganate or concentrated nitric acid : . Finally, the complex of chromium trioxide and 3,5-dimethylpyrazole will selectively oxidize a benzylic methylene group to a carbonyl: R).2-iodoxybenzoic acid in DMSO performs similarly.
Most Common Deprotection Methods
The 2-methoxyethoxymethyl protecting group can be cleaved with a range of Lewis acids, including but not limited to:
- TiCl4 or ZnBr2 in dichloromethane at 0 °C to ambient temperature
- If the solvent of choice is a protic solvent such as methanol, formic acid can be used to cleave MEM group at elevated temperatures
_____________________________
Methoxymethyl is used as a protecting group for alcohols in organic synthesis.
Also Check: Does Elton John Have Biological Children
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
You May Like: Theory Of Everything 2 Music
A Successful Application Of A Silyl Ether Protective Group Strategy
So lets go back to our second example. How could we get this sequence to work? Lets protect the free alcohol as a silyl ether and follow along.
There you have it. All we needed to get our desired reaction to work was a way of masking the OH until we were done performing our surgery on the other half of the molecule.
Common Amine Deprotection Methods
- Mix the protected carbamate to be deprotected with 3 M hydrochloric acid in ethyl acetate for 30 min at ambient temperature
- Heat the carbamate in a mixture of aqueous hydrochloric acid and toluene at 65 °C
- Dissolving desired protected compound in a 50/50 mix of dichloromethane and trifluoroacetic acid
Don’t Miss: What Are Dyes In Chemistry
A Better Way To Do It: Silyl Ethers
Fortunately a very clever solution has been devised. Instead of making a typical ether , we form a silyl ether . Its even easier to form than a normal ether, and shares the property of being inert to many types of reaction conditions. In most introductory courses the most common silyl ether used is trimethylsilyl although there are others
The main advantage of silyl ethers is that theyre easily cleavable. The Si-F bond is unusually strong even stronger than Si-O. Addition of a source of fluoride ion will lead to cleavage of Si-O bonds without affecting the rest of the molecule. A typical source of fluoride ion is the salt tetrabutylammonium fluoride .
Are there other protecting groups for alcohols? You betcha. For more information, see Note 2.
Use Of Protecting Groups
However, 3 can not be converted to 4 using the same two-reaction sequence.
In 3, the most acidic hydrogen atom is not the alkynyl hydrogen but the hydrogen atom in the alcohol group. Consequently, treatment of 3 with the base results in the base deprotonating the alcohol group in 3 giving an alkoxide ion, which reacts with the substrate yielding 5, not 4, as the organic product.
In order to convert 3 to 4 using the methodology employed to convert 1 to 2, the alcohol group in 3 must first be removed temporarily. One way to do so is to convert the alcohol group into a silyl either group.
In 6, the most acidic hydrogen is the alkynyl hydrogen. Treatment of 6 with ¯NH2, followed by the substrate results in 7.
Replacement of the silyl either group in 7 with the alcohol group yields 4.
In the overall reaction , the silyl ether is said to act as the protecting group of the alcohol group reaction is known as the protection step and reaction the deprotection step.
You May Like: Does Kamala Harris Have Children Of Her Own
Summary: Protecting Groups For Alcohols
This post barely scratches the surface of protecting groups for alcohols. Protecting groups are used for alcohols in a variety of different situations, far beyond the SN2 examples we covered here. For instance, when we talk about Grignard reagents, well see that they cant be formed in the presence of alcohols, so we have to protect them. Another example might be if you wanted to selectively oxidize one of two different alcohols in a molecule. We can add more posts on this topic as we go along.
Youll notice that the vast majority of molecules you encounter in Org 1 and Org 2 have only one important functional group. Its very rare that youll be given a substitution reaction, for example, that has two nucleophiles of comparable strength. Learning how to deal with molecules that have more than one key functional group is, in my opinion, where Org 2 ends and Org 3 begins.
Its at that point that you need to learn understand the relative reactivity of different functional groups, their compatibility with different reagents, and also how to plan a synthesis such that only one key functional group will participate in the reaction.
Protecting Groups In Organic Synthesis
One of the major problems in organic synthesis is the suppression of unwanted side reactions. Frequently the desired reaction is accompanied by reaction at other parts of the molecule, especially when more than one functional group is present. Functional groups usually are the most reactive sites in the molecule, and it may be difficult or even impossible to insulate one functional group from a reaction occurring at another. Therefore any proposed synthesis must be evaluated at each step for possible side reactions that may degrade or otherwise modify the structure in an undesired way. To do this will require an understanding of how variations in structure affect chemical reactivity. Such understanding is acquired through experience and knowledge of reaction mechanism and reaction stereochemistry.
To illustrate the purpose and practice of protecting groups in organic synthesis, let us suppose that the synthesis of cis-2-octene, which we outlined in Section 13-7, has to be adapted for the synthesis of 5-octyn-1-ol. We could write the following:
However, the synthesis as written would fail because the alkyne is a weaker acid than the alcohol /11%3A_Alkenes_and_Alkynes_II_-_Oxidation_and_Reduction_Reactions._Acidity_of_Alkynes/11.08%3A_Terminal_Alkynes_as_Acids” rel=”nofollow”> Section 11-8), and the alkynide anion would react much more rapidly with the acidic proton of the alcohol than it would displace bromide ion from carbon:
You May Like: Intermediate Algebra Final Exam Practice
Other Protecting Groups For Alcohols
You may wonder why not use a regular ether instead of a silyl ether. And that is a great question because alcohols can be converted into ether quite easily. The problem, however, is that it is difficult to cleave off the ether group. Ethers are quite stable and in fact, they are used as a solvent for organolithium reactions. Even for the Grignard reaction lab, you will most likely use diethyl ether.
This is an important factor to consider since you dont want your protecting group to be labile under certain conditions but, on the other hand, you need to be able to get it off when the reaction is finished.
A good alternative to the silyl ethers with such qualities is the group called tetrahydropyranyl . It forms a tetrahydropyranyl ether which is stable under basic conditions but can be cleaved with an acid.
The reaction starts by activating the dihydropyran ring which is then attacked by the alcohol:
Acetals are common protecting groups for aldehydes and ketones as well. You can read more about them here.
Last but not least, benzyl ethers represent another common way of protecting the alcohol functional group.
The benzyl group is usually stable under acidic and basic conditions and are cleaved by catalytic hydrogenation with H2 over Pd/C. And because of this, it is not suitable for many reactions that involve a double or a triple bond.