Examples Of Cohesive In A Sentence
cohesivecohesivecohesive WSJcohesive Better Homes & Gardenscohesive clevelandcohesive Los Angeles Timescohesive House Beautifulcohesive Essencecohesive EW.comcohesive Chron
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘cohesive.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Waters High Heat Capacity
The capability for a molecule to absorb heat energy is called heat capacity, which can be calculated by the equation shown in the figure. Waters high heat capacity is a property caused by hydrogen bonding among water molecules. When heat is absorbed, hydrogen bonds are broken and water molecules can move freely. When the temperature of water decreases, the hydrogen bonds are formed and release a considerable amount of energy. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie, or 4.184 Joules. As a result, it takes water a long time to heat and a long time to cool. In fact, the specific heat capacity of water is about five times more than that of sand. This explains why the land cools faster than the sea.
\displaystyle=\frac}}}.
Why Does Water Have High Cohesion
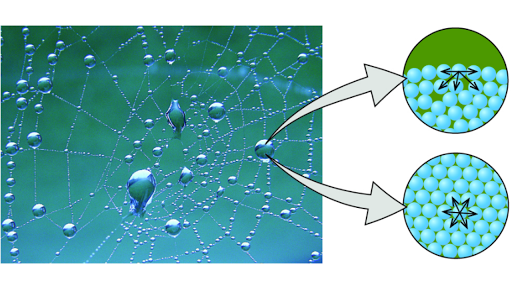
Cohesion refers to the attraction of molecules for other molecules of the same kind, and water molecules have strong cohesive forces thanks to their ability to form hydrogen bonds with one another. Thus, the water molecules at the surface form stronger interactions with the neighbors they do have.
Also Check: Difference Between Electron Geometry And Molecular Geometry
Why Is The High Specific Heat Of Water Important To Earth Quizlet
The high specific heat of water helps to 1) moderate temperature in coastal areas, 2) stabilize ocean temperatures, creating a favorable environment for marine life, 3) because it covers most of the earth it keeps temperature fluctuations within the limits for life, 4) helps organisms (that are made primarily out of
Waters Cohesive And Adhesive Properties
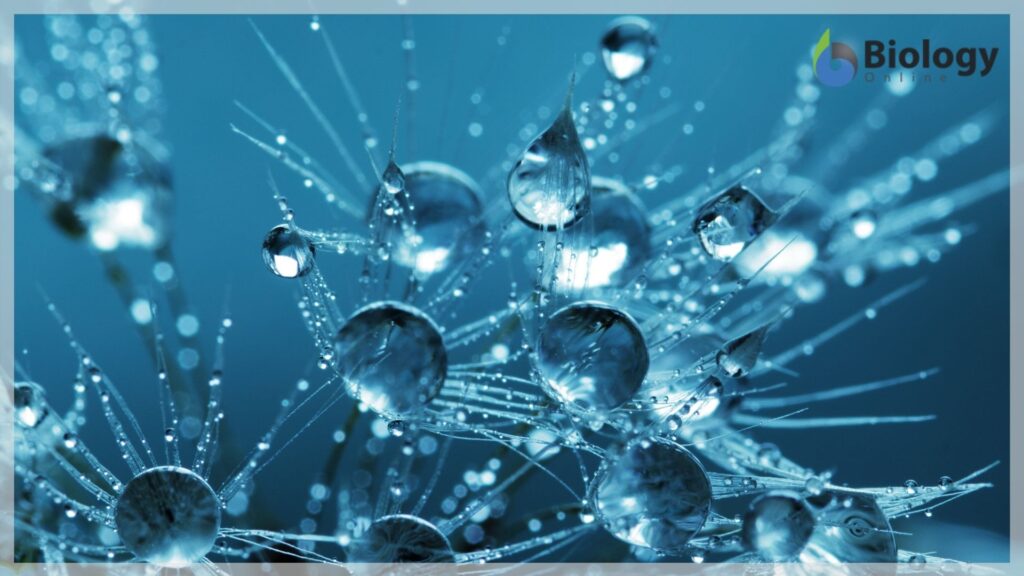
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other , keeping the molecules together at the liquid-gas interface, although there is no more room in the glass.
Cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This is also why water forms droplets when placed on a dry surface rather than being flattened out by gravity. When a small scrap of paper is placed onto the droplet of water, the paper floats on top of the water droplet even though paper is denser than the water. Cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. Its even possible to float a needle on top of a glass of water if it is placed gently without breaking the surface tension.
Surface TensionAdhesionCohesion & Adhesion
- This page has no tags.
Read Also: What Was The Geography And Climate Of New England
Why Is Water Sticky
Adhesion makes a water drop a drop.
Water is highly cohesiveit is the highest of the non-metallic liquids. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and electricity are involved at a more detailed level to make this possible. More precisely, the positive and negative charges of the hydrogen and oxygen atoms that make up water molecules makes them attracted to each other. If you’ve played with bar magnets you will know that the north pole of one magnet will repel the north pole of another magnet, but it will attract the south pole of another magnet. Opposite magnetic poles attract one another much like positively charged atoms attract negatively charged atoms in water molecules.
Examples Of Cohesion In A Sentence
cohesioncohesion Rolling StonecohesionForbescohesionThe New Yorkercohesion New York Timescohesion oregonlivecohesion clevelandcohesion sun-sentinel.comcohesionchicagotribune.com
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘cohesion.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Also Check: Infinite Algebra 1 Properties Of Exponents
How Do You Increase Adhesion
One way that surface roughness aids in adhesion is by increasing the total contact area between the paint and the adherend. If interfacial or intermolecular attraction is the basis for adhesion, increasing the actual area of contact will increase the total energy of surface interaction by a proportional amount.
Chemical Reactions Of Water
Water is directly involved in many chemical reactions to build and break down important components of the cell. Photosynthesis, the process in plants that creates sugars for all life forms, requires water. Water also participates in building larger molecules in cells. Molecules like DNA and proteins are made of repetitive units of smaller molecules. Putting these small molecules together occurs through a reaction that produces water. Conversely, water is required for the reverse reaction that breaks down these molecules, allowing cells to obtain nutrients or repurpose pieces of big molecules.
Figure 4: Water acts as a buffer by releasing or accepting hydrogen atoms.
In conclusion, water is vital for all life. Its versatility and adaptability help perform important chemical reactions. Its simple molecular structure helps maintain important shapes for cells inner components and outer membrane. No other molecule matches water when it comes to unique properties that support life. Excitingly, researchers continue to establish new properties of water such as additional effects of its asymmetrical structure. Scientists have yet to determine the physiological impacts of these properties. Its amazing how a simple molecule is universally important for organisms with diverse needs.
Molly Sargen is a first-year PhD Student in the Biological and Biomedical Sciences Program at Harvard Medical School.
You May Like: Lesson 9.5 Distance In Coordinate Geometry Answers
Molecular Layout Of Liquid Water Molecules
In a water molecule, the two hydrogen atoms align themselves along one side of the oxygen atom, with the result being that the oxygen side has a partial negative charge and the side with the hydrogen atoms has a partial positive charge. Thus when the positive side on one water molecule comes near the negative side of another water molecule, they attract each other and form a bond. This “bipolar” nature of water molecules gives water its cohesive nature, and thus, its stickiness and clumpability .
About Adhesion And Cohesion
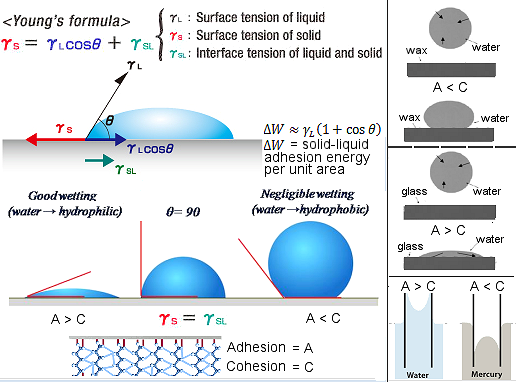
Adhesion forces can be one of the electrostatic forces’ results exerted on various substances. Simultaneously, the cohesive forces are associated with the Van der Waals forces and hydrogen bonding that cause liquids like water to withstand the separation.
When a glass surface is poured with water, the forces of both adhesive, cohesive act on the water’s surface. Also, a strong adhesive force tends the liquid to spread over the surface on the other side, a strong, cohesive force is responsible for forming water droplets on the water surface.
Both the adhesion and cohesion forces vary in their strengths. For example, if the cohesion forces between water molecules are stronger compared to the adhesion forces between them, then the individual molecules present in them will attract towards each other hence resulting in settling. If the adhesion forces of the water surfaces are stronger than the water molecules’ cohesion forces, the water tends to disperse then.
Also Check: Who Are Paris Jackson’s Biological Parents
Water Supports Cellular Structure
Water also has an important structural role in biology. Visually, water fills cells to help maintain shape and structure . The water inside many cells creates pressure that opposes external forces, similar to putting air in a balloon. However, even some plants, which can maintain their cell structure without water, still require water to survive. Water allows everything inside cells to have the right shape at the molecular level. As shape is critical for biochemical processes, this is also one of waters most important roles.
Figure 2: Water impacts cell shape. Figure 3: Phospholipid bilayers.
Buffers Ph Acids And Bases
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper, paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale . Therefore, the more hydrogen ions present, the lower the pH conversely, the fewer hydrogen ions, the higher the pH.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0. Anything below 7.0 is acidic, and anything above 7.0 is alkaline. The blood in your veins is slightly alkaline . The environment in your stomach is highly acidic . Orange juice is mildly acidic , whereas baking soda is basic .
Read Also: What Is Figure Ground Perception Psychology
Biological Roles Of Water: Why Is Water Necessary For Life
figures by Daniel Utter
Water makes up 60-75% of human body weight. A loss of just 4% of total body water leads to dehydration, and a loss of 15% can be fatal. Likewise, a person could survive a month without food but wouldnt survive 3 days without water. This crucial dependence on water broadly governs all life forms. Clearly water is vital for survival, but what makes it so necessary?
Water Properties Photo Gallery
Water Science School HOME Water Properties topics
I used to wake up in a cold sweat because I could not get the concepts of water adhesion and cohesion clear in my mind. If you have that problem, too, then read on to learn about these important properties of water…
Cohesion: Water is attracted to waterAdhesion: Water is attracted to other substances
Adhesion and cohesion are water properties that affect every water molecule on Earth and also the interaction of water molecules with molecules of other substances. Essentially, cohesion and adhesion are the “stickiness” that water molecules have for each other and for other substances.
A water drop is composed of water molecules that like to stick together-an example of the property of cohesion. In the picture of pine needles above, the water droplets are stuck to the end of the pine needles-an example of the property of adhesion. Also noticeable in this picture is the effect that gravity has on the water drops. Gravity is working against both adhesion and cohesion, trying to pull the water drop downward. Adhesion and cohesion are winning the battle so far, as the drops are sticking to the pine needles.
Don’t Miss: Algebra 1 Eoc Test Answers
Why Is Adhesion Important In Life
Adhesion is a water property which affects the living and noon-living things in the environment. Adhesive property of water allows water to stick to non-water molecules. In plants Water moves against gravity due to its adhesive property and allows transport of water from the roots to the shoots and leaves.
Water Is An Excellent Solvent
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solventa substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt mixed in water, the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
Recommended Reading: Does Mj Have Any Biological Kids
Water Is The Universal Solvent
As a polar molecule, water interacts best with other polar molecules, such as itself. This is because of the phenomenon wherein opposite charges attract one another: because each individual water molecule has both a negative portion and a positive portion, each side is attracted to molecules of the opposite charge. This attraction allows water to form relatively strong connections, called bonds, with other polar molecules around it, including other water molecules. In this case, the positive hydrogen of one water molecule will bond with the negative oxygen of the adjacent molecule, whose own hydrogens are attracted to the next oxygen, and so on . Importantly, this bonding makes water molecules stick together in a property called cohesion. The cohesion of water molecules helps plants take up water at their roots. Cohesion also contributes to waters high boiling point, which helps animals regulate body temperature.
Waters States: Gas Liquid And Solid
The formation of hydrogen bonds is an important quality of liquid water that is crucial to life as we know it. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids, and since living things have a high water content, understanding these chemical features is key to understanding life. In liquid water, hydrogen bonds are constantly formed and broken as the water molecules slide past each other. The breaking of these bonds is caused by the motion of the water molecules due to the heat contained in the system. When the heat is raised as water is boiled, the higher kinetic energy of the water molecules causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas . On the other hand, when the temperature of water is reduced and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding . This makes ice less dense than liquid water, a phenomenon not seen in the solidification of other liquids.
Phases of matter: See what happens to intermolecular bonds during phase changes in this interactive.
Ice Density: Hydrogen bonding makes ice less dense than liquid water. The lattice structure of ice makes it less dense than the freely flowing molecules of liquid water, enabling it to float on water.
Recommended Reading: 4.5 Practice B Geometry Answers
Cell Adhesion In Cancer
Cell adhesion is commonly disrupted in cancer. When cells in a tumor have reduced cohesive properties, they can come loose from the tissue and enter the circulatory system. This is how cancer cells can migrate to other sites in the body, a process called metastasis. For example, CAMs called cadherins are frequently found to be deregulated in breast cancer.
Relationship And The Meaning Of Cohesion And Adhesion
In general, cohesion and adhesion forces exist together you can find these respective forces in various activities and processes. Consider the example, meniscus, which is a
liquid surface curvature stored in a tube or container, is caused by both adhesion and cohesion. The attraction force between the edges of liquid and the container wall is called adhesion. The attraction force between the water molecules, which makes the liquid surface curved in the middle, is given cohesion.
Also, the meniscus shape is decided by these forces. If the cohesion force, which exists between the liquid molecules, is more than that of adhesion force existing between the liquid and the tube’s inner surface, the meniscus shape will be convex. For example, mercury, filled in a glass tube. Similarly, if adhesion is more than that of cohesion, the meniscus will be concave. For example, water, filled in a glass tube. The surface will be horizontal if the cohesion is equal to adhesion.
Let us suppose you spill some water on a surface. If the adhesive force is strong, then the water will get absorbed soon by the surface, and also it will go wet. Whereas, if the cohesive force is strong, there will be more attraction between the water molecules than between the surface and water molecules. So, the surface absorbs less water.
Don’t Miss: Exponent Rules Worksheet Algebra 2
Waters Heat Of Vaporization
Water in its liquid form has an unusually high boiling point temperature, a value close to 100°C. As a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to transform one gram of liquid water into water vapor, an energy requirement called the heat of vaporization. Water has a heat of vaporization value of 40.65 kJ/mol. A considerable amount of heat energy is required to accomplish this change in water. This process occurs on the surface of water. As liquid water heats up, hydrogen bonding makes it difficult to separate the water molecules from each other, which is required for it to enter its gaseous phase . As a result, water acts as a heat sink, or heat reservoir, and requires much more heat to boil than does a liquid such as ethanol , whose hydrogen bonding with other ethanol molecules is weaker than waters hydrogen bonding. Eventually, as water reaches its boiling point of 100° Celsius , the heat is able to break the hydrogen bonds between the water molecules, and the kinetic energy between the water molecules allows them to escape from the liquid as a gas. Even when below its boiling point, waters individual molecules acquire enough energy from each other such that some surface water molecules can escape and vaporize this process is known as evaporation.