Gram Atomic Mass And Gram Molecular Mass
The gram atomic mass of an element is the mass of one mole of that element. Similarly, the gram molecular mass of a compound refers to the mass of a single mole of the compound. Therefore, the gram atomic mass of hydrogen is approximately 1.007g and the gram molecular mass of water is approximately 18.015g.
Solution For Problem 92p Chapter 4
Introductory Chemistry | 5th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Introductory Chemistry | 5th Edition
How many neutrons are in an atom with each atomic number and mass number?
Z = 28, A = 59
Z = 92, A = 235
Z = 21, A = 46
Z = 18, A = 42
Z = 28, A = 59
Number of neutrons =A-Z
= 59-28=31
ISBN: 9780321910295
Since the solution to 92P from 4 chapter was answered, more than 1435 students have viewed the full step-by-step answer. The full step-by-step solution to problem: 92P from chapter: 4 was answered by , our top Chemistry solution expert on 05/06/17, 06:45PM. The answer to ?How many neutrons are in an atom with each atomic number and mass number? Z = 28, A = 59 Z = 92, A = 235 Z = 21, A = 46 Z = 18, A = 42 is broken down into a number of easy to follow steps, and 38 words. Introductory Chemistry was written by and is associated to the ISBN: 9780321910295. This full solution covers the following key subjects: atom, atomic, mass, Neutrons. This expansive textbook survival guide covers 19 chapters, and 2046 solutions. This textbook survival guide was created for the textbook: Introductory Chemistry, edition: 5.
Other solutions
History Of Atomic Weight Determinations
As a part of his research on atoms, John Dalton determined a number of atomic weights of elements in the early 1800s. Atomic weights were the basis for the periodic table that Mendeleev developed. Originally all atomic weights were based on a comparison to hydrogen, which has an atomic weight of one. After the discovery of the proton, scientists assumed that the weight of an atom was essentially that of the protonselectrons were known to contribute almost nothing to the atomic weight of the element.
This approach worked until we learned how to determine the number of protons in an element. We then saw that the atomic weight for an element was often twice the number of protons . The discovery of the neutron provided the missing part of the picture. The atomic mass now known to be the sum of the protons and neutrons in the nucleus.
Don’t Miss: What Is Figure Ground Perception Psychology
Finding The Mass Number
The best place to look for an element’s atomic mass number is in the periodic table. It’s displayed under the symbol for the element. You might be mystified by the fact that in many versions of the periodic table, this number contains a decimal fraction, which you wouldn’t expect if it was derived simply by adding protons and neutrons.
The reason for this is that the number displayed is the relative atomic weight, which is derived from all the naturally occurring isotopes of the element weighted by the percentage of each that occurs. Isotopes are formed when the number of neutrons in an element is more or less than the number of protons. Some of these isotopes, such as carbon-13, are stable, but some are unstable and decay over time to a more stable state. Such isotopes, such as carbon-14, are radioactive.
Virtually all elements have more than one isotope, so each has an atomic mass that contains a decimal fraction. For example, the atomic mass of hydrogen listed in the periodic table is 1.008, that for carbon is 12.011 and that for oxygen is 15.99. Uranium, with an atomic number of 92, has three naturally occurring isotopes. Its atomic mass is 238.029. In practice, scientists usually round mass number to the nearest integer.
Characteristics Of Mass Number
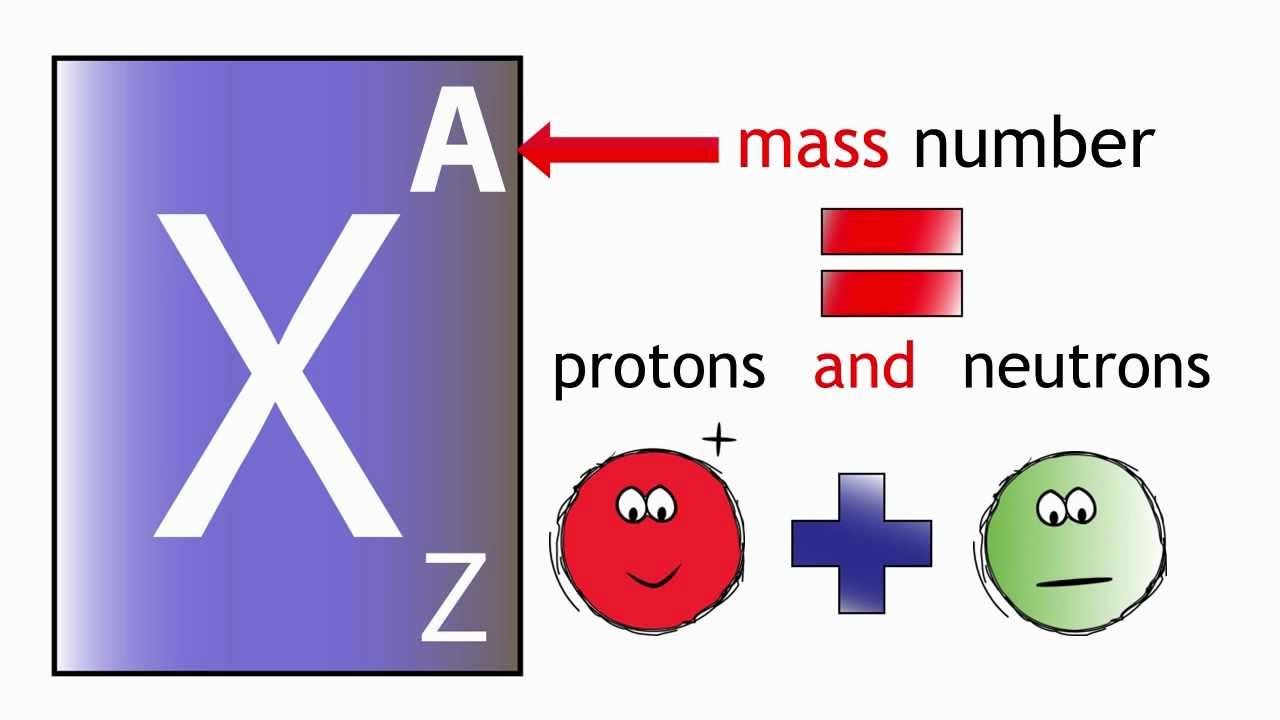
The mass numbers is an integer equal to the sum of the numbers of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom.
The mass number is often denoted using a capital letter A. Contrast this with the atomic number, which is simply the number of protons.
Also Check: 6 Major Branches Of Chemistry
Mass Number Changes In Radioactive Decay
Different types of radioactive decay are characterized by their changes in mass number as well as atomic number, according to the radioactive displacement law of Fajans and Soddy. For example, uranium-238 usually decays by alpha decay, where the nucleus loses two neutrons and two protons in the form of an alpha particle. Thus the atomic number and the number of neutrons each decrease by 2 , so that the mass number decreases by 4 the result is an atom of thorium-234 and an alpha particle (42He2+
-
e
Beta decay is possible because different isobars have mass differences on the order of a few electron masses. If possible, a nuclide will undergo beta decay to an adjacent isobar with lower mass. In the absence of other decay modes, a cascade of beta decays terminates at the isobar with the lowest atomic mass.
Another type of radioactive decay without change in mass number is emission of a gamma ray from a nuclear isomer or metastable excited state of an atomic nucleus. Since all the protons and neutrons remain in the nucleus unchanged in this process, the mass number is also unchanged.
Numbers Of Isotopes Per Element
Of the 80 elements with a stable isotope, the largest number of stable isotopes observed for any element is ten . No element has nine or eight stable isotopes. Five elements have seven stable isotopes, eight have six stable isotopes, ten have five stable isotopes, nine have four stable isotopes, five have three stable isotopes, 16 have two stable isotopes , and 26 elements have only a single stable isotope . In total, there are 252 nuclides that have not been observed to decay. For the 80 elements that have one or more stable isotopes, the average number of stable isotopes is 252/80 = 3.15 isotopes per element.
Also Check: Does Elton John Have Biological Children
Use Of Nuclear Properties
- A technique similar to radioisotopic labeling is radiometric dating: using the known half-life of an unstable element, one can calculate the amount of time that has elapsed since a known concentration of isotope existed. The most widely known example is radiocarbon dating used to determine the age of carbonaceous materials.
- Several forms of spectroscopy rely on the unique nuclear properties of specific isotopes, both radioactive and stable. For example, nuclear magnetic resonance spectroscopy can be used only for isotopes with a nonzero nuclear spin. The most common nuclides used with NMR spectroscopy are 1H, 2D, 15N, 13C, and 31P.
- Mössbauer spectroscopy also relies on the nuclear transitions of specific isotopes, such as 57Fe.
- Radionuclides also have important uses. Nuclear power and nuclear weapons development require relatively large quantities of specific isotopes. Nuclear medicine and radiation oncology utilize radioisotopes respectively for medical diagnosis and treatment.
Is Mole Fraction Equal To Partial Pressure
In a mixture, the partial pressure of each gas is proportional to the mole fraction. The pressure exerted by each gas in the gas mixture is independent of the pressure exerted by all other gases present in the gas mixture.
To learn more about the mole concept and other related concepts, such as the law of definite proportions, register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Is The Mole Concept
The mole concept is a convenient method of expressing the amount of a substance. Any measurement can be broken down into two parts the numerical magnitude and the units that the magnitude is expressed in. For example, when the mass of a ball is measured to be 2 kilograms, the magnitude is 2 and the unit is kilogram.
When dealing with particles at an atomic level, even one gram of a pure element is known to contain a huge number of atoms. This is where the mole concept is widely used. It primarily focuses on the unit known as a mole, which is a count of a very large number of particles.
Atomic Mass Of Elements
On the physical scale of atomic mass or weight, the natural oxygen atom is taken as the reference. But natural oxygen was found to be the mixture of three isotopes of mass number 16, 17, and 18 with the percentage of abundance 993575, 0.039, and 0.204 respectively. The true mass of natural oxygen = + + = 16.00447. The exact number 16 as the relative mass of oxygen represents the new scale of atomic weights. In the chemical scale of the atomic weight or masses, the exact number 16 is taken as the reference.
Read Also: Math Age Problems
Characterization Of Proteins Containing Selenocysteine
The atomic mass of selenium is 47 amu greater than that of sulfur. Hence, mass spectrometry is a useful tool for demonstrating the replacement of sulfur by selenium. Matrix-assisted laser desorption ionization time-of-flight mass spectral analysis shows that the mass of folded wild-type RNase A is 13,820 Da and that of folded C110U RNase A is 13,865 Da . Not only are the experimental values in excellent agreement with the predicted values, but also the mass difference of 45 amu is close to that expected for the replacement of sulfur by selenium.
Fig. 5. MALDI-TOF mass spectra of semisynthetic wild-type RNase A and C110U RNase A .
A critical measure of the successful ligation and folding of a selenium-containing protein is the demonstration of function. For C11QU RNase A, that demonstration involves measuring ribonucleolytic activity. The kcat/Km for the cleavage of 6-FAM~dArU2~6-TAMRA22 is × 107 M 1 sec 1 for wild-type RNase A and × 107 M 1 sec 1 for C110U RNase A. The similarity of these values indicates that selenium can replace sulfur with minimal perturbation to function and structure.
Therald Moeller, … Clyde Metz, in, 1980
Nature Of The Particles
The mole is essentially a count of particles. Usually the particles counted are chemically identical entities, individually distinct. For example, a solution may contain a certain number of dissolved molecules that are more or less independent of each other. However, in a solid the constituent particles are fixed and bound in a lattice arrangement, yet they may be separable without losing their chemical identity. Thus the solid is composed of a certain number of moles of such particles. In yet other cases, such as diamond, where the entire crystal is essentially a single molecule, the mole is still used to express the number of atoms bound together, rather than a count of multiple molecules. Thus, common chemical conventions apply to the definition of the constituent particles of a substance, in other cases exact definitions may be specified.The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams.
Don’t Miss: Law Of Figure And Ground
Solved Examples On The Mole Concept
Some solved example questions on the mole concept are provided in this subsection.
Q.1: How many moles of iron are present in a pure sample weighing 558.45 grams?
A.1: The molar mass of iron is 55.845 g/mol. Therefore, the number of moles of iron in the pure sample weighing 558.45 grams is:
= 10 moles.
Q.2: How many molecules of water are present in 36 grams of water?
A.2: The molar mass of water is 18 . Therefore, 36 grams of water makes up a total of 2 moles. Each mole has 6.022*1023 water molecules. The total number of H2O molecules in 36 grams of water is: 12.044*1023
Q.3: How many grams of carbon can be found in 1 mole of carbon dioxide?
A.3: 1 mole of CO2 contains 1 mole of carbon and 2 moles of oxygen. The molar mass of carbon is 12.0107 g/mol. Therefore, 1 mole of CO2 contains 12.01 grams of carbon and 32 grams of oxygen.
Atomic Mass Of Isotopes
The atomic mass of an isotope is determined mainly by its mass number . Small corrections are due to the binding energy of the nucleus , the slight difference in mass between proton and neutron, and the mass of the electrons associated with the atom, the latter because the electron:nucleon ratio differs among isotopes.
The mass number is a dimensionless quantity. The atomic mass, on the other hand, is measured using the atomic mass unit based on the mass of the carbon-12 atom. It is denoted with symbols “u” or “Da” .
The atomic masses of naturally occurring isotopes of an element determine the atomic mass of the element. When the element contains N isotopes, the expression below is applied for the average atomic mass m
x N }_=m_x_+m_x_+…+m_x_}
where m1, m2, …, mN are the atomic masses of each individual isotope, and x1, …, xN are the relative abundances of these isotopes.
Don’t Miss: Segment Addition Postulate Worksheet Answers
Atomic Number Of Elements
Atomic number of chemical elements in chemistry is the number of protons of an atom by which the elements are arranged in the periodic table. The modern periodic system is formed on the basis of atomic number and electronic configuration of the atom but Mendeleev classification is based on the atomic weight or mass of an element. In learning chemistry, a good correlation between electromagnetic spectrum and atomic number indicated that an element is characterized by its atomic number, not atomic weight. The trends and properties like ionization energy, electron affinity, shielding effect of an atom are better to describe by its atomic number.
Atomic Number Orbital Energy Levels
When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Orbitals are the name for these sections. Sublevels are made up of orbitals with the same energy. A maximum of two electrons can be found in each orbital.
The most common way of showing the arrangement of electrons in an atom is to draw diagrams like those shown in the diagram.
To write down the numbers of electrons in each energy level. The atomic number of an element tells us how many electrons there are in the atoms. For example, the atomic number of carbon is 6 giving us six electrons as 2,4. So an atom with the atomic number 12 has an electronic structure 2, 8, 2, with two electrons in the inner energy level, then eight in the next energy level and two in the outer highest energy level. The simplest way to understand these arrangements is to look at lots of examples of them.
Don’t Miss: Grade 6 Fsa Warm-ups Answer Key
How Can You Determine The Mass Of A Chemical
Often a student will need to weigh out a chemical for an experiment. If he or she uses a watch glass , the weight of the watch glass must be determined first. Then the solid is added to the glass and the weight of the glass plus the solid is measured. The balance reading will be the total of the glass plus the chemical.
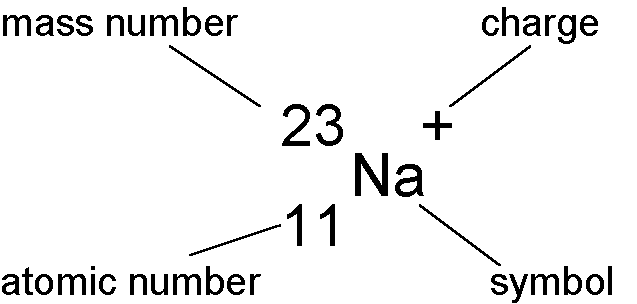
Explain Atomic Number And Mass Number Of Element
To know about mass number, Firstly we should know about atomic mass, atoms and molecules. What is atomic mass?
Atomic weight or Atomic mass
- Mass of an atom is called as atomic mass. Total 118 elements are present in periodic table. But Its very difficult to find out atomic mass of each atom.
- The atomic mass of an element is the average mass of the atom of that element, it is measured by atomic mass unit which is amu.
- So its unit is amu or u.
- In other words, we can say, it is the average atomic weight of an element, measured in units of atomic mass, also called Dalton.
- The atomic weight is the average of the weight of all isotopes of an element, and the mass of each isotope multiplied by the frequency is a specific isotope.
- Atomic mass is also called atomic weight, but the term mass is more accurate.
- Carbon-12 has an atomic mass of 1 to 12 times of 1.992646547 x 10-23 gram, and has an atomic mass of 12 units.
- On this scale, one unit of atomic mass is equivalent to 1.660539040 X 10-24g.
- As you know that all matters are made up of atoms and molecules in this universe.
- Although all molecules are made up of atoms thats why atoms are fundamental unit of matters.
- Atom word is coming from Atomos which means indivisible. But later on, it found that atoms are not indivisible. It contains proton, neutron and electron.
- Generally, protons are positive in charge, electrons are negative, and neutrons are neutral.
You May Like: Michael Jackson Kids Biological
Mass Number And Isotopic Mass
The mass number gives an estimate of the isotopic mass measured in atomic mass units . For 12C, the isotopic mass is exactly 12, since the atomic mass unit is defined as 1/12 of the mass of 12C. For other isotopes, the isotopic mass is usually within 0.1 u of the mass number. For example, 35Cl has a mass number of 35 and an isotopic mass of 34.96885. The difference of the actual isotopic mass minus the mass number of an atom is known as the mass excess, which for 35Cl is 0.03115. Mass excess should not be confused with mass defect which is the difference between the mass of an atom and its constituent particles .
There are two reasons for mass excess: