What Is A Basic Definition Of Element
An element is a substance that cannot be separated into simpler substances through chemistry. An element is also an important component of something or a natural habitat. Element has many other senses as a noun.
In chemistry, an element is something that cannot be broken down any further. If you have taken a chemistry class, youve likely seen the periodic table, which displays all the known chemical elements. The study and measuring of elements is one of the central focuses of the scientific field of chemistry. For example, water is made of the elements hydrogen and oxygen. We can split water into hydrogen and oxygen, but we cannot use chemistry to split oxygen or hydrogen into anything else.
- Real-life examples: The substances we know as carbon, oxygen, nitrogen, calcium, and gold are examples of elements.
- Used in a sentence: Ammonia is made of the elements nitrogen and hydrogen.
Outside of science, an element is a main component or ingredient of something, as bricks would be for a brick wall, for example. The words elemental and elementary are sometimes used in a similar sense to describe things that are the simplest principles or basic components of something.
- Real-life examples: Peanut butter, jelly, and bread are the elements of a PB& J sandwich. Cement and water are elements of concrete. Tires, brakes, and an engine are elements of a functioning vehicle.
- Used in a sentence: Love and trust are elements of a strong relationship.
Elements And Atoms: Elements
What should we make of these two quite different-sounding definitions? After all, the list of elements discovered and characterized by scientists using the older definition are still elements under the newer definition. Even so, the different definitions embody important conceptual differences. The classical definition evokes macroscopic processes of analytical “wet” chemistry, and is silent on the structure of elements the modern definition is a microscopic one, inseparable from an understanding of the structure of matter and of particular details of the atomic nucleus. The alteration in definition reminds us that as knowledge changes, so does the language which expresses that knowledge. Sometimes new words are used to express new ideas sometimes old words are given new meanings.
Key Takeaways: Chemical Element
- A chemical element is a substance consisting of only one type of atom. In other words, all atoms in an element contain the same number of protons.
- The identity of a chemical element cannot be changed by any chemical reaction. However, a nuclear reaction can transmute one element into another one.
- Elements are considered to be the building blocks of matter. This is true, but it’s worth noting atoms of an element consist of subatomic particles.
- There are 118 known elements. New elements may yet be synthesized.
Don’t Miss: What Is Elastic Force In Physics
What Is An Element In Chemistry Definition And Examples
In chemistry, an element is defined as a pure substance composed of atoms that all have the same number of protons in the atomic nucleus. In other words, all atoms of an element have the same atomic number. The atoms of an element cannot be broken into smaller particles by any chemical means. Elements can only be broken into subatomic particles or transmuted into other elements by nuclear reactions. At present, there are 118 known elements.
If atoms of an element carry an electrical charge, they are called ions. Atoms of an element with different numbers of neutrons are called isotopes. Sometimes isotopes also have their own names, but they are still examples of an element. For example: protium, deuterium, and tritium are all isotopes of the element hydrogen. Elements can take different forms called allotropes, but this doesnt change their chemical identity. For example: diamond and graphite are both pure elemental carbon.
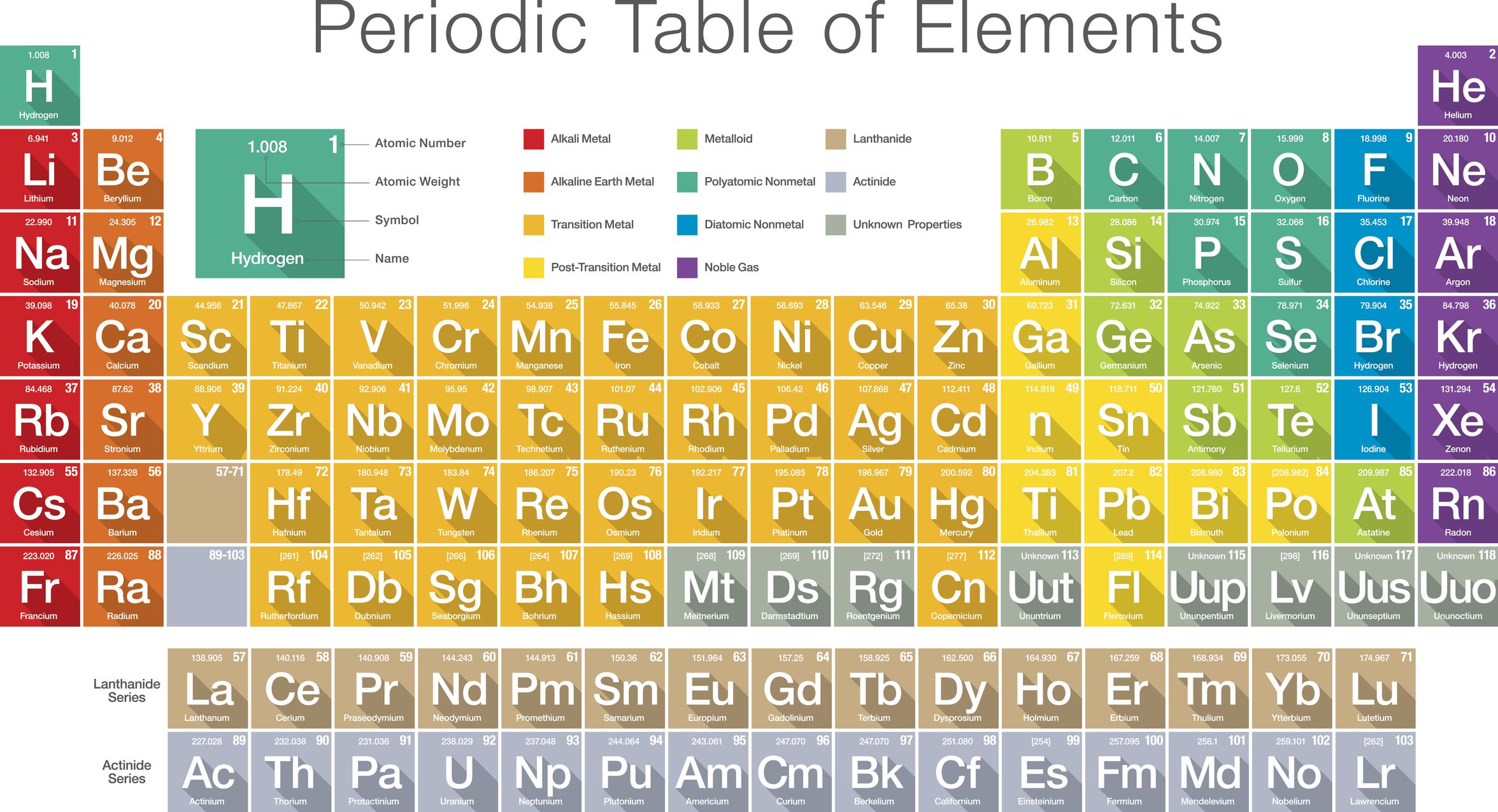
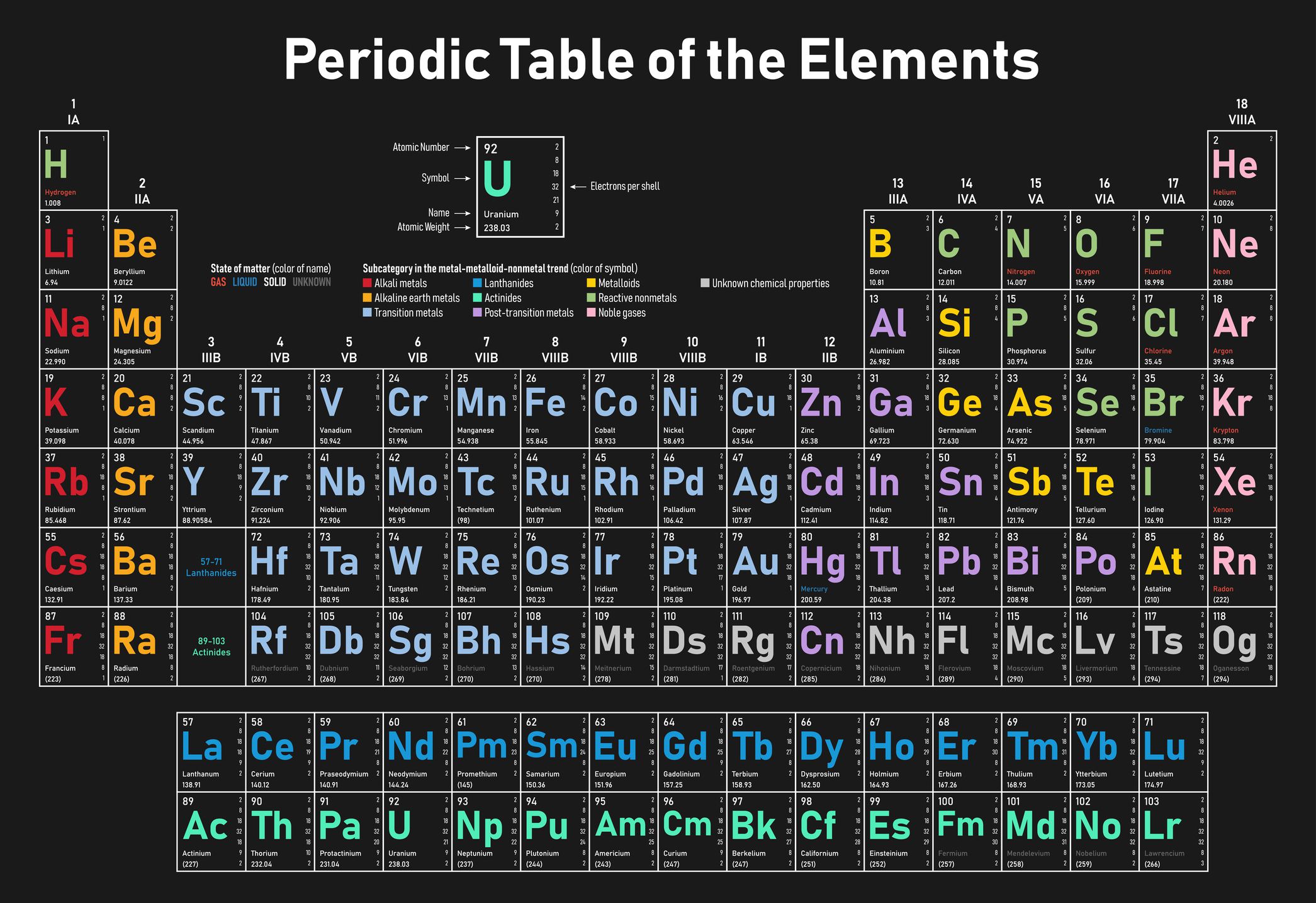
List Of The 118 Known Chemical Elements
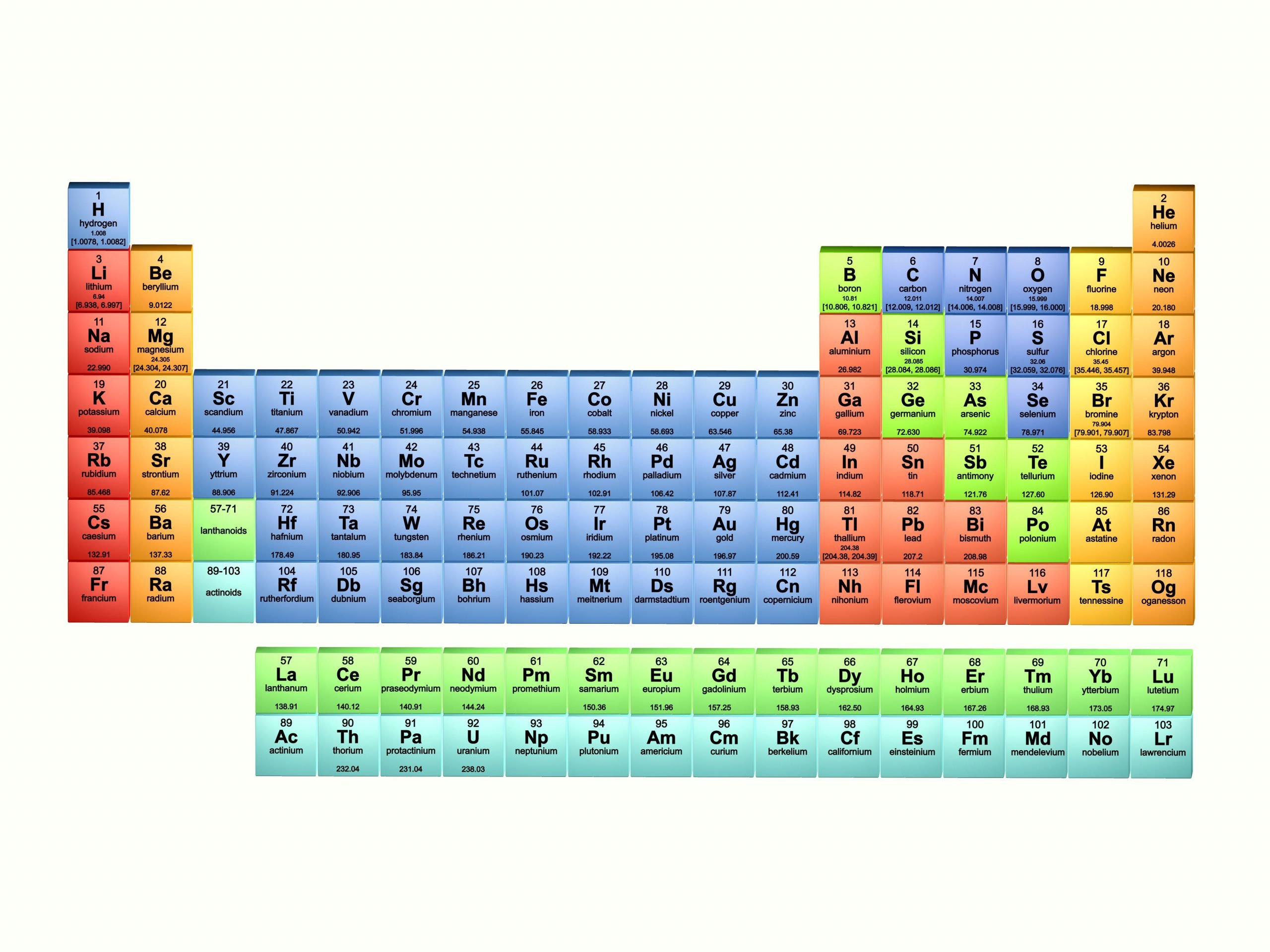
The following sortable table shows the 118 known chemical elements.
- Atomic number, Element, and Symbol all serve independently as unique identifiers.
- Element names are those accepted by IUPAC.
- Symbol column background color indicates the periodic table block for each element: red = s-block, yellow = p-block, blue = d-block, green = f-block.
- Group and period refer to an element’s position in the periodic table. Group numbers here show the currently accepted numbering for older alternate numberings, see Group .
Don’t Miss: What Does Fault Mean In Geography
What Is An Element
The chemical element read as follows:
a species of atoms all atoms with the same number of protons in the atomic nucleus. A pure chemical substance composed of atoms with the same number of protons in the atomic nucleus.
The question of the conceptual nature of the term element represents a rather unique opportunity to examine the relationship that currently exists between chemists and philosophers of chemistry.
Chemical element was first presented by the English scientist Robert Boyle. He defined an element as a substance incapable of decomposition and like a true scientist added the prophetic by any means with which we are now acquainted. Boyles definition comes admirable close to present day theory. Elements have been changed in the laboratories of today, though not by any chemical means.
Where Does Element Come From
The first records of element come from around 1250. It ultimately comes from the Latin elementum, meaning one of the four elements or rudiment.
In early history, it was thought that all of creation was made of four basic things: earth, air, fire, and water. The word elementum referred to these substances.
Today, we know that creation is actually more complicated, but we sometimes still use the word element to refer to these substances, particularly in fantasy stories and other works of fiction.
Also Check: Lewis Dot For Ccl4
Chemically Pure And Isotopically Pure
Chemists and nuclear scientists have different definitions of a pure element. In chemistry, a pure element means a substance whose atoms all have the same atomic number, or number of protons. Nuclear scientists, however, define a pure element as one that consists of only one stable isotope.
For example, a copper wire is 99.99% chemically pure if 99.99% of its atoms are copper, with 29 protons each. However it is not isotopically pure since ordinary copper consists of two stable isotopes, 69% 63Cu and 31% 65Cu, with different numbers of neutrons. However, a pure gold ingot would be both chemically and isotopically pure, since ordinary gold consists only of one isotope, 197Au.
Schools Wikipedia Selection Related Subjects: General Chemistry
A chemical element, often called simply an element, is a substance that cannot be decomposed or transformed into other chemical substances by ordinary chemical processes. All matter consists of these elements and as of 2006, 117 unique elements have been discovered or artificially created. The smallest particle of such an element is an atom, which consists of electrons centered about a nucleus of protons and neutrons.
You May Like: How To Login To Imagine Math
Choose The Right Synonym For Element
element, component, constituent, ingredient mean one of the parts of a compound or complex whole. element applies to any such part and often connotes irreducible simplicity. the basic elements of geometry component and constituent may designate any of the substances or the qualities that enter into the makeup of a complex product component stresses its separate entity or distinguishable character. the components of a stereo system constituent stresses its essential and formative character. the constituents of a chemical compound ingredient applies to any of the substances which when combined form a particular mixture. the ingredients of a cocktail
Origin Of The Elements
Only about 4% of the total mass of the universe is made of atoms or ions, and thus represented by chemical elements. This fraction is about 15% of the total matter, with the remainder of the matter being dark matter. The nature of dark matter is unknown, but it is not composed of atoms of chemical elements because it contains no protons, neutrons, or electrons. .
The 94 naturally occurring chemical elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the Big Bang. This Big Bang nucleosynthesishappened only once the other processes are ongoing. Nuclear fusion inside stars produces elements through stellar nucleosynthesis, including all elements from carbon to iron in atomic number. Elements higher in atomic number than iron, including heavy elements like uranium and plutonium, are produced by various forms of explosive nucleosynthesis in supernovae and neutron star mergers. The light elements lithium, beryllium and boron are produced mostly through cosmic ray spallation of carbon, nitrogen, and oxygen.
In addition to the 94 naturally occurring elements, several artificial elements have been produced by human nuclear physics technology. As of 2021, these experiments have produced all elements up to atomic number 118.
Read Also: Algebra 1 Honors Eoc Practice Test
Regions Of Element Synthesis
A discussion of how the present chemical composition of the universe has arisen brings to light two distinct questions: what was the initial chemical composition and what alterations have occurred since creation. Ideally, by working backwards, the initial composition can be deduced from the present composition and a life-history, but this approach is overambitious. The initial composition predicted by simple cosmological theory can then be tested for compatibility with present observations. Element production in the universe as a whole can be discussed first production in stars and other objects in the Galaxy is treated in the sections that follow.
Examples Of Chemistry In A Sentence
chemistrychemistrychemistrychemistry clevelandchemistry Washington Postchemistry Detroit Free Presschemistry CNNchemistrySan Diego Union-Tribunechemistry Chronchemistry BostonGlobe.comchemistryUSA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘chemistry.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Read Also: Which Founding Contributors To Psychology Helped Develop Behaviorism
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
What Are The Main Elements
The Five Base Elements are Fire, Earth, Metal, Water, and Wood. In a state of continuous interaction and flux with each other these elements are known as different types of energy. Not only does The Five Elements mean Fire, Earth, Water, Metal, and Wood. We also apply to motion, transition, and growth.
Read Also: My Hrw Com Algebra 1
What Is A Proton In Chemistry Definition
A proton is a subatomic particle found in the nucleus of every atom. The particle has a positive electrical charge, equal and opposite to that of the electron. The number of protons in an element’s nucleus is called the atomic number.
Likewise, people ask, what is a proton simple definition?
proton. The definition of a proton is a particle with a positive charge that is in the nucleus of an atom. An example of a proton is the single proton in the nucleus of a hydrogen atom.
Also, what is a proton and its function? Function in the AtomThe protons inside an atom’s nucleus help bind the nucleus together. They also attract the negatively charged electrons, and keep them in orbit around the nucleus. The number of protonsin an atom’s nucleus determines which chemical element it is.
Moreover, what is a proton definition for kids?
Kids Definition of proton: a very small particle that exists in the nucleus of every atom and has a positive charge of electricity.
Why is a proton important?
A proton is one of the most important types of subatomic particles. Protons combine with electrons and neutrons to make atoms. Protons are nearly the same size as neutrons and are much larger than electrons. Clouds of electrons orbit the nucleus, attracted by the positive charges of the protons.
You May Like Also
Water Is A Polar Molecule
Note also that the sharing of electrons is not always equal. For example, in a water molecule, the negatively charged electrons spend more time in the vicinity of the heavier oxygen atom.
The net result is that the water molecule has one end that is more negative relative to the other end. Water is therefore a “polar” molecule. We will see that this polarity has important implications for many biological phenomena including cell structure. You may have heard the expression “like dissolves like.” What this means is that polar molecules dissolve well in polar fluids like water. Sugars and salts are polar molecules, and they dissolve in water, because the positive and negative parts of the two types of molecules can distribute themselves comfortably among one another.
You May Like: Geometry Segment Addition Postulate Worksheet
Historical Development Of The Concept Of Element
In the latter part of the Middle Ages, as alchemists became more sophisticated in their knowledge of chemical processes, the Greek concepts of the composition of matter became less satisfactory. Additional elemental qualities were introduced to accommodate newly discovered chemical transformations. Thus, sulfur came to represent the quality of combustibility, mercury that of volatility or fluidity, and salt that of fixity in fire . These three alchemical elements, or principles, also represented abstractions of properties reflecting the nature of matter, not physical substances.
The important difference between a mixture and a chemical compound eventually was understood, and in 1661 the English chemist Robert Boyle recognized the fundamental nature of a chemical element. He argued that the four Greek elements could not be the real chemical elements because they cannot combine to form other substances nor can they be extracted from other substances. Boyle stressed the physical nature of elements and related them to the compounds they formed in the modern operational way.
Element Production In The Universe As A Whole
Hydrogen and helium are overwhelmingly the most abundant elements in the objects of which there is direct knowledge, and, as some buildup of heavy elements occurs in stars, the working hypothesis is usually adopted that the initially created matter contained only light elements.
Observations of distant galaxies suggest that the universe is expanding and that galaxies may have been very close together at some time. In the big-bang theory it is assumed that the universe was created at that time, 13.8 billion years ago, and that at its creation the universe was very hot as well as very dense. Nuclear reactions in the early stages of the expansion lead to a rather well-defined initial chemical composition for the universe.
The big-bang theory not only predicts that all objects, except those in which the helium could have been destroyed, should have a minimum of about 25 percent helium but that the microwave radiation should have a particular distribution with frequency known as the Planck form. Recent determinations of the primordial helium abundance have converged on a value of 25 percent, and observations with the Cosmic Background Explorer satellite have shown the frequency distribution of the microwave background radiation to be a perfect Planck form.
Read Also: Geometry Basics Unit 1 Answers
What Is The Definition Of An Element
Element definition in chemistry is Any pure substance that is made entirely of one type of atom and cannot be broken by chemical means. It has a set number of protons in it and changing the number of protons of an element changes the type of element entirely. The number of protons in the nuclei is unique for each element and is known as its atomic number. There are around 118 known elements to date. Since elements are made up of atoms, let us first see some basic terms that you will need to know to understand elements.
Elements Molecules And Compounds
An element consists of only one type of atom. A molecule is composed of two or more atoms joined together by chemical bonds. Some molecules are examples of elements, such as H2, N2, and O3. A compound is a type of molecule consisting of two or more different atoms joined by chemical bonds. All compounds are molecules, but not all molecules are compounds.
Note: The IUPAC makes no distinction between molecules and compounds, defining them as a pure substance formed by a fixed ratio of two or more atoms sharing chemical bonds. By this definition, O2 would be an element, a molecule, and a compound. Because of differing definitions, chemistry teachers probably ought to stay away from questions about elements/compounds and simply focus on the 118 elements of the periodic table as examples of elements.
Recommended Reading: Kuta Software Infinite Geometry Volume Of Prisms And Cylinders
Key Takeaways: Chemical Element Definition
- A chemical element is a substance that cannot be further broken down by any chemical reaction.
- Each element has a unique number of protons in its atom. For example, a hydrogen atom has 1 proton, while a carbon atom has 6 protons.
- Varying the number of electrons in an atom of an element produces ions. Changing the number of neutrons produces isotopes.
- There are 118 known elements.