Find The Percent Abundance
To get percent abundance, we will multiply the relative abundance value by 100 and put a percent sign.
As we have now x= 0.76. Simply, multiply by 100 to get percent. So, chlorine-35 is 0.76*100=76%
Now,
; = = 0.24
Multiplying above by 100, we get 24%.;
Therefore, the percent abundance of chlorine-35 is 76% and the percent abundance of chlorine-37 is 24%.;
How Does Isotope Abundance Impact Atomic Weight
Atomic mass depends on the composition of protons and neutrons in an element, with each weighing 1 atomic mass unit . Electrons are an important part of elements as well, but they have such a small mass that they are considered negligible when calculating atomic mass. As both protons and neutrons make up an atoms mass, when an element differs in its number of neutrons, it is impactful on the mass.
Though they sound like synonyms, atomic mass and atomic weight are different. Isotopes impact the value of both. Atomic mass is defined as the mass of an individual atom of an element. This is solely the calculation of the weight of protons and neutrons in amu. Atomic weight on the other hand is the weighted average of all of the isotopes of an element that exist. This is where isotope abundance comes in. Though there may be many naturally occurring isotopes of an element, they do not exist in equal amounts. There are many isotopes that occur much more commonly than others, and therefore have a greater impact on the atomic weight. If given the atomic mass of the isotopes of an element as well as their relative abundances, we can follow simple steps to calculate the atomic weight.
Abundance Of Elements In Earth’s Crust
This graph illustrates the relative abundance of the chemical elements in Earth’s upper continental crust.
Many of the elements shown in the graphic are classified into categories:
Note that there are two breaks where the unstable elements technetium and promethium would be. These are very rare, as on Earth they are only produced through the fission of heavy radioactive elements . Both elements have been identified spectroscopically in the atmospheres of stars, where they are produced by ongoing nucleosynthetic processes. There are also breaks where the six noble gases would be as they are found in the Earth’s crust due to decay chains from radioactive elements and are therefore not included. The six very rare, highly radioactive elements are not included, as their natural abundances are too low to have been accurately measured.
Oxygen and silicon are notably common; they form several common silicate minerals.
“Rare earth” element abundances
Read Also: What Kind Of Math Is On The Ged Test
The Core Difference Lies In The Nucleus: Neutrons
To get to the core of why isotopes have varying mass but similar chemical properties, we have to examine their atomic structure.
Isotopes have the same number of protons but different number of neutrons packed into the nucleus. Since the neutron has a relative mass of 1, having more neutrons will increase the relative mass of an isotope. For example, hydrogen-3 is the heaviest isotope of hydrogen as it has the most number of neutrons.
But neutrons are electrically neutral. Having more of them does not change the overall positive charge of the nucleus, which will attract the same number of electrons. With the same electronic configuration, isotopes form the same type of bond and have similar chemical properties. This is evident in the isotopes of hydrogen, as they all have a single electron each.
Isotopes are defined as atoms of the same element with the same number of protons but different number of neutrons. Consequently, they have the same atomic number but different mass number.
Abundance Of The Chemical Elements
The abundance of the chemical elements is a measure of the occurrence of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by the mass-fraction ; by the mole-fraction ; or by the volume-fraction. Volume-fraction is a common abundance measure in mixed gases such as planetary atmospheres, and is similar in value to molecular mole-fraction for gas mixtures at relatively low densities and pressures, and ideal gas mixtures. Most abundance values in this article are given as mass-fractions.
For example, the abundance of oxygen in pure water can be measured in two ways: the mass fraction is about 89%, because that is the fraction of water’s mass which is oxygen. However, the mole-fraction is about 33% because only 1 atom of 3 in water, H2O, is oxygen. As another example, looking at the mass-fraction abundance of hydrogen and helium in both the Universe as a whole and in the atmospheres of gas-giant planets such as Jupiter, it is 74% for hydrogen and 2325% for helium; while the mole-fraction for hydrogen is 92%, and for helium is 8%, in these environments. Changing the given environment to Jupiter’s outer atmosphere, where hydrogen is diatomic while helium is not, changes the molecular mole-fraction , as well as the fraction of atmosphere by volume, of hydrogen to about 86%, and of helium to 13%.
Read Also: Who Are Paris Jackson’s Biological Parents
Membership Of The Sponsoring Body
Membership of the IUPAC Inorganic Chemistry Division Committee for the period 2014â2015 was as follows:
President: J. Reedijk ; Secretary: M. Leskelä ; Vice President: L. R. Ãhrström ; Past President: R. D. Loss; Titular Members: T. Ding ; M. Drábik ; E. Y. Tshuva ; D. Rabinovich ; T. Walczyk ; M. E. Wieser ; Associate Members: J. Buchweishaija ; J. Garcia-Martinez ; P. Karen ; A. Kilic ; K. Sakai ; R.-N. Vannier ; National Representatives: F. Abdul Aziz ; L. Armelao ; A. Badshah ; V. Chandrasekhar ; J. Galamba Correia ; S. Kalmykov ; L. Meesuk ; S. Mathur ; B. Prugovecki ; N. Trendafilova .
Membership of the IUPAC Commission on Isotopic Abundances and Atomic Weights for the period 2014â2015 was as follows:
Chair: J. Meija ; Secretary: T. Prohaska ; Titular Members: W. A. Brand ; M. Gröning ; R. Schönberg ; X.-K. Zhu ; Associate Members: T. Hirata ; J. Irrgeher ; J. Vogl ; National Representatives: P. De Bièvre ; T. B. Coplen ; Ex-officio member: J. Reedijk .
Isotopes Are Atoms Of The Same Element With Different Masses
Atoms of the same element can have different mass. We say that they are isotopes of the same element.
For example, hydrogen has three isotopes with different relative mass, which is indicated by the number behind their name. So hydrogen-1 has a relative mass of 1, while hydrogen-2 has twice the mass. Hydrogen-3 is the heaviest isotope, with a relative mass of 3.
While their relative mass is different, isotopes are like peas in a pod in terms of chemical properties. They react in an almost identical way, making it impossible to distinguish them through reactions. For example, all three isotopes of hydrogen react explosively with oxygen in a 2:1 ratio.
Isotopes of the same element have different physical properties but similar chemical properties.
Don’t Miss: Exponential Growth And Decay Worksheet Answer Key Algebra 1
What Is Percent Abundance
The percentage of a specific isotope that exists in nature is the relative concept of percent abundance in chemistry. Abundance can be calculated by three ways: by mass fraction, by mole fraction and by volume fraction.; The atomic weight indicated on the periodic table for an element constitutes an average mass of all recognized isotopes.
The atomic nuclei only comprise protons and neutrons, each of these having a mass of around 1 atomic mass unit . The atomic weight of each element should also be a whole number. Moreover,;electron weights;are considered negligible and are not involved in the atomic weight of an element. As we know that, when we look at the periodic table, it is mentioned that the most of the elements have a decimal;fraction of their atomic weights.;So, actually, the mass specified for each element is mean of all the isotopes that exist in nature which is. The abundance of each isotope of the element can be determined instantly if we know how often the isotopes have atomic weights.
The recognition;of the element appears the same as the number of neutrons inside the nucleus varies. A modification in the number of neutrons in the nucleus indicates an isotope. There might be zero, one, two, more neutrons in the nuclei depending on the element. For example; Hydrogen has three isotopes. The nucleus of protium or simply hydrogen consists no neutron but only proton, deuterium has a neutron in the nucleus and tritium has two neutrons in the nucleus.
Calculate The Relative Atomic Mass Using The Formula
A = 1×99.97% + 2×0.03%A = 1.0003 1
When we round off the relative atomic mass of hydrogen to the nearest whole number, it is approximately 1. This corroborates with the data booklet, which shows the relative atomic mass of an element below its chemical symbol.
Likewise, the relative atomic mass of chlorine is 35.5 because it consists two isotopes: chlorine-35 and chlorine-37. The lighter isotope is more common, with a relative abundance of 75%. This explains why the average value of 35.5 is closer to 35 than to 37.
Study more leh:
Read Also: What Is Figure Ground Perception Psychology
What If Elements Have More Than Two Isotopes
For example:;
Oxygen has three naturally occurring isotopes 16O,; 17O and; 18O. The average atomic mass of oxygen is 15.9994 amu. Given, the atomic weight of 16O is 15.995 amu,; 17O is 16.999 amu and; 18O is 17.999 amu. Also, 17O has 0.037 percent in nature. What are the other isotopes percent abundances?;
Firstly, we have the abundance of one isotope which is 0.0037. So, the abundances of the other two remaining isotopes is = 0.99963.
Let x be the unknown abundance of 16O and other isotope abundance of 18O be .
Now, modifying equation , we get
* + * + * = 15.9994
or 15.995x 17.999x = 15.9994
or x = 0.9976
So, we get the abundance of 16O is 0.9976 and the abundance of 18O is = 0.00203.;
Hence, the percent abundance of three isotopes are given by;
16O=0.9976*100=99.76%
An Easy Explanation Of How To Find Percent Abundance
According to chemistry principles, isotopes have same atomic number but different mass number. Abundance is defined as the amount of isotope contained in its parent element. This ScienceStruck post tells you how to calculate percent abundance for any element having isotopes.
Like it? Share it!
According to chemistry principles, isotopes have same atomic number but different mass number. Abundance is defined as the amount of isotope contained in its parent element. This ScienceStruck post tells you how to calculate percent abundance for any element having isotopes.
NoteAn element can consist of number of isotopes, and it is necessary to consider all of its isotopes while computing the percent abundance. Consider element carbon , it has 15 isotopes, and it is mandatory to consider all of them.
Every element has atoms which consist of protons, electrons, and neutrons. When an element has more than one form having same number of protons but different number of neutrons in the nucleus, they are called isotopes. They either occur naturally or are artificially produced. The element chlorine has more than 50 isotopes, of which Cl-35 and Cl-37 are two stable isotopes. They have same atomic number, which is 17; however, their mass number is 35 and 37, respectively.
Definition of percentage abundance for X in Y can be best explained in two possible ways as follows:
1. how many percent of Y is X.2. how much of X is in Y
Average mass of an element= +
Read Also: Define Span Linear Algebra
Abundance: Some Isotopes Are More Common Than Others
| isotope of hydrogen | |
|---|---|
| 3 | trace |
Hydrogen-1 is the most common isotope, with a relative abundance of 99.97%. This means that out of 10000 hydrogen atoms in a sample of hydrogen gas, 9997 are hydrogen-1.
On the other hand, hydrogen-3 is highly unstable and can barely exist. It only forms temporarily under very special circumstances, like in a nuclear reactor or when a nuclear weapon explodes. Therefore, it has a relative abundance of close to zero.
Abundance is the percentage of the number of atoms of a particular isotope out of the total number of atoms in a sample of an element.
How To Calculate Percentage Abundance Using Atomic And Isotopic Masses
To calculate percentage abundance, you must recall the atomic mass of an element is calculated by using the formula:
Formula for calculating atomic mass
In the above formula you see fractional abundance.;How do you get that? To get fractional abundance, you usually divide the percentage abundance of each isotope by 100. And when you add all the fractional abundance values of all the isotopes, you will notice they all add up to 1. To calculate the;percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100. Now, lets apply our understanding to solve the following question:
Silver has two stable isotopes: silver-107 and silver-109 . Silver-107 has a mass of 106.90509 amu and silver-109 has a mass of 108.90476 amu. Calculate the percentage abundance of each isotope.
Strategy
To calculate percentage abundance, we must first know the fractional abundance of each isotope. But from the question, we are not given these values, which means we must think of a way of finding them. One way we can find them is to remember that the:
;fractional abundance of isotope 1 plus the fractional abundance of isotope 2 = 1
Setup for calculating fractional abundance
107.8682 amu = 106.90509X amu;;;+;;108.90476 amu;;;;;108.90476X;amu
107.8682 amu 108.90476 amu = 106.90509;X amu 108.90476;X;amu
Next, we subtract like terms
-1.03656
Read Also: What Does Abiotic Mean In Biology
Viral Genomes In Nature
Elements With Two Isotopes
If an element has two isotopes, you can readily set up an equation to determine the relative abundance of each isotope based on the weight of each isotope and the weight of the element listed in the periodic table. If you denote the abundance of isotope 1 by x, the equation is:
W1 x + W2 = We
since the weights of both isotopes must add to give the weight of the element. Once you find , multiply it by 100 to get a percentage.
For example, nitrogen has two isotopes, 14N and 15N, and the periodic table lists the atomic weight of nitrogen as 14.007. Setting up the equation with this data, you get: 14x + 15 = 14.007, and solving for , you find the abundance of 14N to be 0.993, or 99.3 percent, which means the abundance of 15N is 0.7 percent.
Don’t Miss: Ccl4 Angle
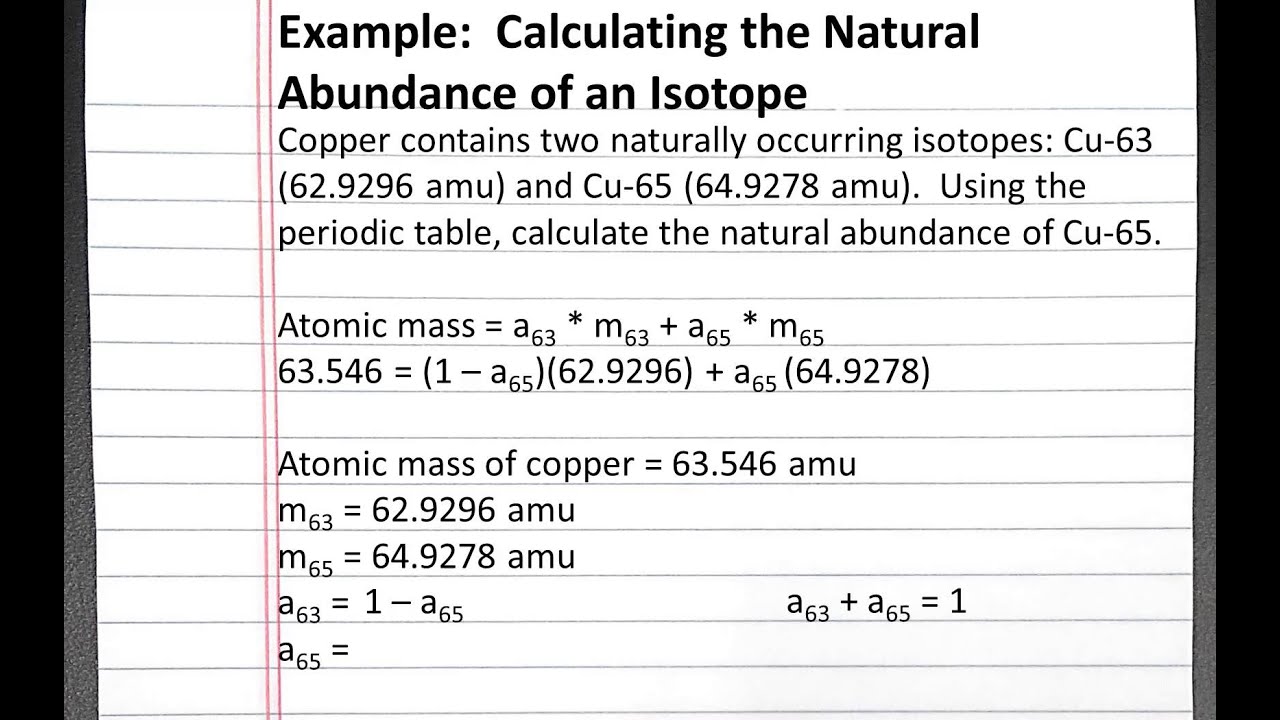
What Is The Natural Abundance Of Potassium
The three naturally occurring isotopes of potassium are $\ce$ ; $\ce$ ; and $\ce$.
The percent natural abundances of $\ce$ and $\ce$ are $93.2581\%$ and $6.7302\%$ respectively.
a) What is the natural abundance of $\ce$?
b) Determine the isotopic mass of $\ce$.
So I started to utilize this equation:
$\mathrm$
But then I realized that I did not have some values. $\ce$ is the only isotope which has two values. $\ce$ only has the mass weight, however, if I were to multiply $\ce$’s mass with would that help me arrive at an answer?
Also, would that answer be accurate since it only takes into account that there are only to values that add up to 100%.
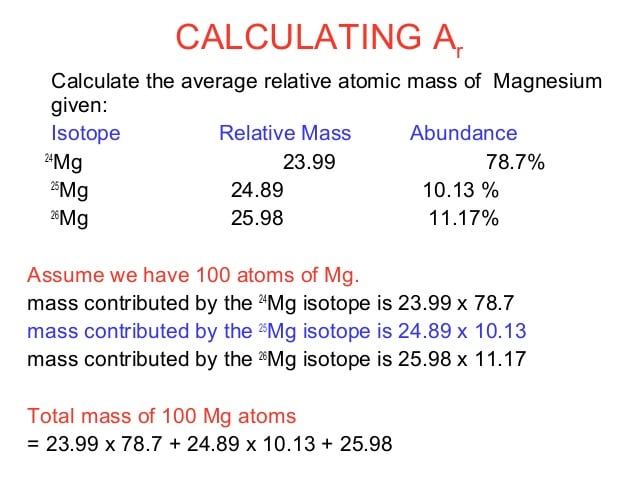
Using Isotope Abundance To Calculate Atomic Weight
As stated previously, the number of isotopes and their percent abundance are all that are needed to calculate the atomic weight of an element. We can start by using magnesium as an example. Magnesium has three naturally occurring isotopes: 24Mg, 25Mg, and 26Mg. Each isotope has an abundance of 78.70 %, 10.13%, and 11.17%, respectively. The atomic mass of each isotope is usually very close to each isotope value. In this example, the mass of each isotope is 23.985 amu, 24.985 amu, and 25.982 amu respectively.
Now that we have all of the information about mass and abundance, we can calculate the atomic weight of magnesium. If you have trouble visualizing all of the values, you can organize them in a table to make your information more clear.
| Isotope | |
| 25.982 | 11.17% |
We start by multiplying each isotopes mass by its abundance. This can be done in two ways. First, we can directly multiply the mass by the percent:
23.985 amu = 1887.6 amu
On the other hand, we can change the percent to a decimal out of one and then multiply by the mass. This can be done by dividing the percent by 100.
23.985 amu = 18.876 amu
With both of these methods, the next step is to repeat for the other isotopes and add the values together.
Method 1:
23.985 amu + 24.985 amu + 25.982 amu =
1887.6 amu + 253.09 amu + 290.21 amu = 2430.90 amu
Method 2:
23.985 amu + 24.985 amu + 25.982 amu = 24.3090 amu
2430.90 amu/100= 24.3090 amu
Don’t Miss: Age Word Problems With Solutions