Covalent Vs Metallic Bond In Nitinol
In the absence of hybridization, s-orbital leads to the formation of metallic bond whereas d-orbital leads to the formation of covalent bond . This means the d-orbital of Ti is potentially capable of forming covalent bond with the d-orbital of Ni, providing the energy level of the two d-orbital from Ti and Ni are not too far apart. Now, let us represent this energy level difference as Ed . Analogously, there would be Ed and Ed . From Fig. 5 we see that the order of magnitude for the three Eds should be
Heavy Metals In Science
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In science, a heavy metal is a metallic element which is toxic and has a high density, specific gravity or atomic weight. However, the term means something slightly different in common usage, referring to any metal capable of causing health problems or environmental damage.
How Strong Is A Metallic Bond
Metals have a high attraction force between their atoms. To overcome it, a lot of energy is required. Metals have high boiling points as a result, with tungsten being particularly high.
To learn more about metallic bonds and other important types of chemical bonds , register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Also Check: Application Of Linear Algebra In Daily Life
Roman Numerals In Chemistry
As if chemistry is not complicated enough for some of us, we sometimes run into Roman numerals in chemistry formulas. For example, should you write copper chloride or copper chloride? Is it iron sulfide or iron sulfide? Why are Roman numerals used in chemistry? How do you know when an element needs a Roman numeral?
Physical Properties Of Metals
- All the metals are good conductors of heat and electricity. Cooking utensils and irons are made up of metals as they are good conductors of heat.
- Ductility is the ability of the material to be stretched into a wire. This ability allows metals to be drawn into wires and coupled with their durability, find applications as cable wires and for soldering purposes. Because Metal can be drawn into wires we can say that metals are ductile.
- Malleability is the property of substances which allows them to be beaten into flat sheets. Aluminium sheets are used in the manufacturing of Aircrafts because of their lightweight and strength. Other metal sheets are used in automobile industries, for making utensils, etc. Therefore, metals are malleable.
- Metals are sonorous because they produces a deep or ringing sound when struck with another hard object.
- Usually, all the metals have a shiny appearance but these metals can also be polished to have a shiny appearance.
Recommended Reading: How To Find Speed In Physics
High Melting And Boiling Points
As a result of powerful metallic bonding, the attractive force between the metal atoms is quite strong. In order to overcome this force of attraction, a great deal of energy is required. This is the reason why metals tend to have high melting and boiling points. The exceptions to this include zinc, cadmium, and mercury .
The metallic bond can retain its strength even when the metal is in its melt state. For example, gallium melts at 29.76oC but boils only at 2400oC. Therefore, molten gallium is a non volatile liquid.
Metallic Character And Periodic Table Trends
There are trends in metallic character as you move across and down the periodic table. Metallic character decreases as you move across a period in the periodic table from left to right. This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell.
Metallic character increases as you move down an element group in the periodic table. This is because electrons become easier to lose as the atomic radius increases, where there is less attraction between the nucleus and the valence electrons because of the increased distance between them.
Don’t Miss: What Is Time In Physics
Using The Periodic Table To Predict Metallic Character
The most common homework question about metallic character is identifying which element is most or least metallic. Answer these questioning by comparing element positions on the periodic table.
- Elements on the left side of the periodic table are more metallic than elements in the right side of the periodic table. The exception is hydrogen, which is a nonmetal under ordinary conditions.
- Elements near the bottom of the table are more metallic than elements near the top of the table. Remember that the lanthanides are in group 3 and period 6, while the actinides are in group 3 and period 7.
- Metals are more metallic than metalloids, which are more metallic than nonmetals.
Water Vapor Capture And Dehumidification
MOFs have been demonstrated that capture water vapor from the air. In 2021 under humid conditions, a polymer-MOF lab prototype yielded 17 liters of water per kg per day without added energy.
MOFs could also be used to increase energy efficiency in room temperature space cooling applications.
When cooling outdoor air, a cooling unit must deal with both the air’s sensible heat and latent heat. Typical vapor-compression-air-conditioning units manage the latent heat in air through cooling fins held below the dew point temperature of the moist air at the intake. These fins condense the water, dehydrating the air and thus substantially reducing the air’s heat content. The cooler’s energy usage is highly dependent on the cooling coil’s temperature and would be improved greatly if the temperature of this coil could be raised above the dew point. This makes it desirable to handle dehumidification through means other than condensation. One such means is by adsorbing the water from the air into a desiccant coated onto the heat exchangers, using the waste heat exhausted from the unit to desorb the water from the sorbent and thus regenerate the desiccant for repeated usage. This is accomplished by having two condenser/evaporator units through which the flow of refrigerant can be reversed once the desiccant on the condenser is saturated, thus making the condenser the evaporator and vice versa.
You May Like: What Is Rationalization In Psychology
Examples Of Metallic In A Sentence
metallicmetallicmetallicmetallicmetallic Glamourmetallic Harper’s BAZAARmetallic Voguemetallic PEOPLE.commetallic USA TODAYmetallic Country Livingmetallic SPINmetallic Better Homes & Gardens
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘metallic.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Key Takeaways: Metallic Character
- Metallic character is the set of properties associated with metals.
- These properties include metallic luster, formation of cations, high electrical and thermal conductivity, and malleability.
- Metallic character is a periodic table trend. The elements with the most metallic character are on the left side of the periodic table .
- Francium is the element with the highest metallic character.
Read Also: What Are Human Characteristics In Geography
What Is The Difference Between Metallic Bonding And Ionic Bonding
Ionic bonds involve the transfer of electrons between two chemical species. They arise from a difference in the electronegativities of the bonded atoms. On the other hand, metallic bonds are formed when a rigid, definite lattice of metal cations share a sea of delocalized valence electrons. However, both these types of bonding involve electrostatic forces of attraction.
What Are We Eating As A Nation

The graph above indicates some trends in the U.S. diet over a thirty-year period. By observing the direction our eating habits are going, steps can be taken to help prevent bad eating habits and decrease problems such as high blood pressure and heart attacks.
Development of the periodic table has helped organize chemical information in many ways. We can now see trends among properties of different atoms and make predictions about the behavior of specific materials.
Don’t Miss: Holt Algebra 1 End Of Course Assessment Answers
What Is Metallic Character
Metallic character is the name given to the set of chemical properties associated with elements that are metals. These chemical properties result from how readily metals lose their electrons to form cations .
Physical properties associated with metallic character include metallic luster, shiny appearance, high density, high thermal conductivity, and high electrical conductivity. Most metals are malleable and ductile and can be deformed without breaking. Many metals are hard and dense.
Metals display a range of values for these properties, even for elements that are considered highly metallic. For example, mercury is a liquid at room temperature rather than a hard solid. It also has a lower electrical conductivity value than other metals. Some of the noble metals are brittle rather than malleable. At the same time, these metals are still shiny and metallic-looking, plus they form cations.
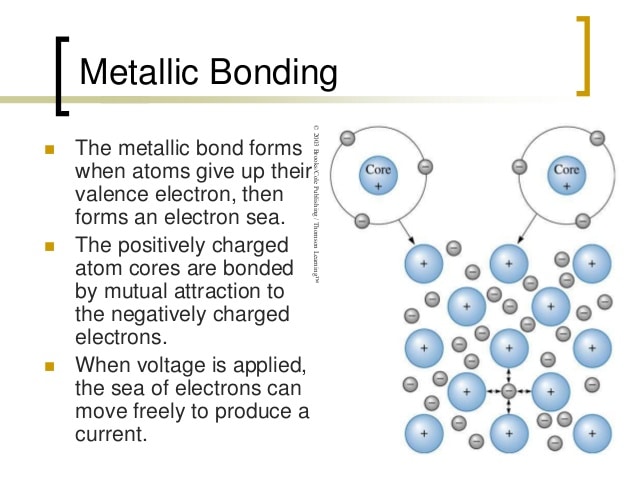
What Is A Metallic Bond
Metallic bond is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions.
Metallic bonding is a type of chemical bonding and is responsible for several characteristic properties of metals such as their shiny lustre, their malleability, and their conductivities for heat and electricity.
Don’t Miss: What Is Quantitative Techniques In Geography
Recognizing Elements With Metallic Character
You can use the periodic table to predict whether or not an element will display metallic character, even if you don’t know anything about it. Here’s what you need to know:
- Metallic character is displayed by metals, which are all on the left-hand side of the periodic table. The exception is hydrogen, which is a nonmetal under ordinary conditions. Even hydrogen behaves as a metal when it’s a liquid or solid, but you should consider it nonmetallic for most purposes.
- Elements with metallic character occur in certain groups or columns of elements, including the alkali metals, alkaline earth metals, transition metals , and the basic metals. Other categories of metals include base metals, noble metals, ferrous metals, heavy metals, and precious metals. The metalloids display some metallic character, but this group of elements also has nonmetallic properties.
Chemical Properties Of Metals
Don’t Miss: What Is An Intervening Obstacle In Human Geography
What Transition Metals Dont Need Roman Numerals
The above list of transition metals contain three exceptions in terms of Roman numeral assignment. These are aluminum, zinc and silver. These metals exist in only one ion therefore, they cannot give away more than one ion. Thus, even though they are transition metals, aluminum, zinc and silver are only and never require Roman numerals written after their names.
Horizontal Trend: Metallic Character Across A Period
The metallic character decreases from left to right across a period in the periodic table. As the number of protons increases, the attractive electrostatic force between the nucleus and electrons increases, and the electrons are held tightly. Hence, it is not easy for atoms to give away electrons. The atoms towards the right of the periodic table prefer to accept electrons.
Recommended Reading: Which Hormone Is Called Biological Clock
What Is An Element And How Are Elements Classified
An element is a substance that is made entirely from one type of atom. For example, the element hydrogen is made from atoms containing a single proton and a single electron. The number of elements discovered has increased, making it difficult to remember the behavior and properties of these elements.
What Is Metal Or Non
A metal is a component, material, or alloy that is usually solid, transparent, shiny, and has good conductivity in terms of electrical and thermal. Metals are usually malleable, which ensures that they can be indefinitely hammered or pressed without fracturing or cracking, as well as fusible or ductile
Also Check: What Is Visual Information Processing In Psychology
Alloys And Metallic Character
Although the term metallic character is typically applied to pure elements, alloys may also display metallic character. For example, bronze and most alloys of copper, magnesium, aluminum, and titanium typically display a high level of metallicity. Some metallic alloys consist purely of metals, but most also contain metalloids and nonmetals yet retain the properties of metals.
Heavy And Light Metals
A heavy metal is any relatively dense metal or metalloid. More specific definitions have been proposed, but none have obtained widespread acceptance. Some heavy metals have niche uses, or are notably toxic some are essential in trace amounts. All other metals are light metals.
Recommended Reading: How To Find Precision In Chemistry
Bulk Properties Of Metals
Metals have several properties that they are known for. Some of them are conductivity, malleability, ductility, luster, high melting point, and high boiling point. Metals exist in the form of a solid structure, but they can be easily deformed. The properties of metals in free state are due to the arrangement of electrons in the metallic bond. Since valence electrons are free, delocalized, mobile and not associated with any particular atom, it is possible to explain several properties of metals.
Base Noble And Precious Metals
In chemistry, the term base metal is used informally to refer to a metal that is easily oxidized or corroded, such as reacting easily with dilute hydrochloric acid to form a metal chloride and hydrogen. Examples include iron, nickel, lead, and zinc. Copper is considered a base metal as it is oxidized relatively easily, although it does not react with HCl.
The term noble metal is commonly used in opposition to base metal. Noble metals are resistant to corrosion or oxidation, unlike most base metals. They tend to be precious metals, often due to perceived rarity. Examples include gold, platinum, silver, rhodium, iridium, and palladium.
In alchemy and numismatics, the term base metal is contrasted with precious metal, that is, those of high economic value.A longtime goal of the alchemists was the transmutation of base metals into precious metals including such coinage metals as silver and gold. Most coins today are made of base metals with low intrinsic value in the past, coins frequently derived their value primarily from their precious metal content.
Recommended Reading: What Is Atomic Radius In Chemistry
Metallic Character Trend On The Periodic Table
Both metallic and nonmetallic character are periodic table trends. Metallic character increases moving down a periodic table group and decreases moving across a period. Moving down a group, atoms add electron shells so the atomic radius increases and it takes less energy to remove electrons. Moving across a period , the number of protons increases but the number of electron shells remains the same. This increases the effective nuclear force on electrons and makes it more difficult to remove them.
Metallic character follows the atomic radius periodic table trend. As you might expect, it is opposite the trends for ionization energy, electron affinity, and electronegativity. Increasing ionization energy, electron affinity, and electronegativity are associated with non-metallic character.
How Metal Elements Differ From Nonmetal Elements
There are currently 118 known elements on the periodic table, many of which are classified as either a metal or nonmetal. The former are found on the left side of the periodic table, whereas the latter are found on the right side of the periodic table. Aside from their placement on the periodic table, though, there are several key differences between metal and nonmetal elements.
You May Like: Trek Madone 9.9 Geometry
What Is The Definition Of Metallic Property
An elements metallic properties refer to its propensity to behave like the elements that are classified as metals in the periodic table. This depends on the set of chemical properties commonly associated with the metallic elements, specifically the ability of an element to lose its outer valence electrons.
The metallic character of an element depends on how readily it can lose its electrons. This results in the element exhibiting chemical properties like the ability to be reduced, the ability to form ionic chlorides and basic oxides and the ability to displace hydrogen from dilute acids. Metallic character decreases from left to right and increases from top to bottom on the periodic table. This means that the most metallic elements are located on the lower left part of the table.
Metallic And Nonmetallic Character

Metallic character refers to the level of reactivity of a metal. Metals tend to lose electrons in chemical reactions, as indicated by their low ionization energies. Within a compound, metal atoms have relatively low attraction for electrons, as indicated by their low electronegativities. By following the trend summary in the figure below, you can see that the most reactive metals would reside in the lower left portion of the periodic table. The most reactive metal is cesium, which is not found in nature as a free element. It reacts explosively with water and will ignite spontaneously in air. Francium is below cesium in the alkali metal group, but is so rare that most of its properties have never been observed.
Reactivity of metals is based on processes such as the formation of halide compounds with halogens, and how easily the element displaces hydrogen from dilute acids.
The metallic character increases as you go down a group. Since the ionization energy decreases going down a group , the increased ability for metals lower in a group to lose electrons makes them more reactive. In addition, the atomic radius increases going down a group, placing the outer electrons further away from the nucleus and making that electron less attracted by the nucleus.
Also Check: How Do We Know That Clocks Are Hungry Math Answer