How Do You Find The Atomic Radius In Chemistry
Atomic radiusatomsatomic radiusatomsatomic radiusatoms
. Accordingly, what is an atomic radius in chemistry?
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the center of the nucleus to the boundary of the surrounding shells of electrons. Three widely used definitions of atomic radius are: Van der Waals radius, ionic radius, and covalent radius.
Furthermore, what is the atomic radius of all elements? Atomic Radius of the elements
| Helium |
|---|
| 171 pm |
Keeping this in consideration, where is the atomic radius on an element?
The atomic radius of a chemical element is the distance from the centre of the nucleus to the outermost shell of the electron.
Why is atomic radius important?
The size of atoms is important when trying to explain the behavior of atoms or compounds. One of the ways we can express the size of atoms is with the atomic radius . This data helps us understand why some molecules fit together and why other molecules have parts that get too crowded under certain conditions.
Atomic Radius Decreases From The Bottom Left To The Upper Right Hand Corner Of The Periodic Table
When looking at the entire periodic table, it is easy to see which elements have the largest radii and which element have the smallest radii. Because the elements with the most energy shells and the least amount of electrons in those energy shells have the largest radii, it is easy to determine that Ununennium has the largest atomic radius of all the known elements. This is because it has the most energy shells and the least amount of electrons in its outermost shell .
Similarly, it can be determined that Helium will have the smallest atomic radius of all the elements. This is because it has the fewest energy shells and enough electrons to fill its energy shell . Additionally, because the number of electrons in the outermost shell is equivalent to the number of protons in a He atom, the forces between these two types of atomic particles will ensure that the radius of this atom is very small.
What Is A Atomic Radius In Chemistry
atomic radiuschemicalatomsradiusatom’s
. Furthermore, what is atomic radius used for?
This Term Describes the size of an AtomBut It’s Not PreciseD. Atomic radius is a term used to describe the size of an atom. However, there is no standard definition for this value. The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der Waals radius.
Beside above, what is the atomic radius of all elements? Atomic Radius of the elements
| Helium |
|---|
| 171 pm |
Furthermore, how do you determine atomic radius?
Measures of atomic radiusThe radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance. As you can see from the diagrams, the same atom could be found to have a different radius depending on what was around it.
Where is the atomic radius on an element?
The atomic radius of a chemical element is the distance from the centre of the nucleus to the outermost shell of the electron.
Recommended Reading: Whatever Happened To Beth Thomas Child Of Rage
Atomic Radius Of Chemical Elements
It must be noted, atoms lack a well-defined outer boundary. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Therefore, there are various non-equivalent definitions of atomic radius.
- Van der Waals radius. In principle, Vana der Waals radius is half the minimum distance between the nuclei of two atoms of the element that are not bound to the same molecule.
- Ionic radius. An ionic radius is one-half the distance between the nuclei of two ions in an ionic bond.
- Covalent radius. Covalent radius is the nominal radius of the atoms of an element when covalently bound to other atoms.
- Metallic radius. A metallic radius is one-half the distance between the nuclei of two adjacent atoms in a crystalline structure, when joined to other atoms by metallic bonds.
How To Use Van Der Waals Forces To Calculate Atomic Radius
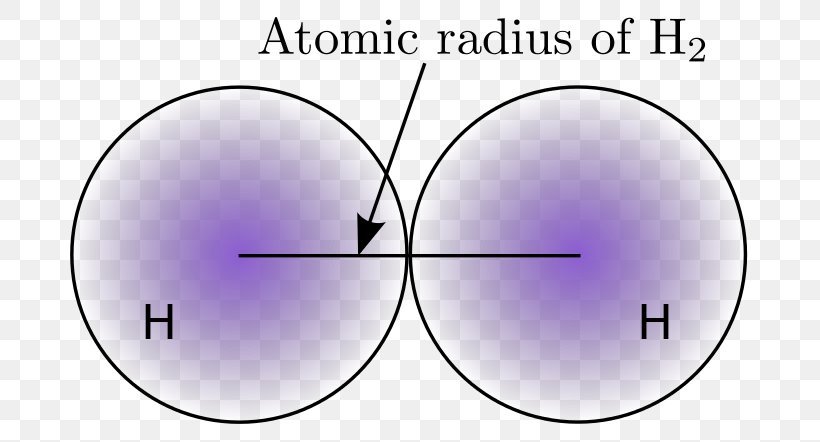
The forces that draw two dipoles together can be used to calculate the radius of an atom. To do this, it is necessary to use two atoms of the same element. It is also best to use atoms that have high polarizability because these atoms also have higher Van der Waal forces.
It is fairly easy to determine how polarizable an atom is. The more electrons an atom has, the more likely it is to be able to become polarized. Additionally, the more electrons an atom has, the higher the Van der Waal forces are between these nuclei.
To calculate the atomic radius of two dipoles of the same element, simply find the nuclei of these atoms and measure the distance in between them. Once you have this number, divide by 2. The resulting number is the atomic radius.
This method is so effective because the Van der Waal forces ensure that the atoms will be drawn as close as they can without merging. Once this happens, measuring from the nucleus of the first atom to the nucleus of the second atom is effectively the same as measuring the diameter of an atom. Because the radius is half the diameter, simply dividing by 2 solves the equation.
You May Like: Age Problems Worksheet
Using Two Atoms Of The Same Element That Are Bonded
It is also possible to determine the atomic radius of an element by looking at two atoms of the same element that are bonded together through a covalent bond. This covalent bond means that the two atoms can share electrons at one point in time, the majority of the atoms could be in the first atoms energy shield, in the next moment the majority of the atoms could be in the second atoms energy shield.
Although these two atoms share a covalent bond, it can be assumed that the bond will not be able to alter the distance between the nucleus and the theoretical outermost point of an electron. This means that all that needs to be done is to measure the distance between the two nuclei and then divide that number by 2. The resulting number would be the atomic radius of the element.
What Is The Unit Of Atomic Radius
5/5AngstromsNanometer
Thereof, what are SI units of atomic radius?
Atomic radii are often measured in angstroms , a non-SI unit: 1 Å = 1 × 10 10 m = 100 pm.
Additionally, what does atomic radius mean? The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the center of the nucleus to the boundary of the surrounding shells of electrons. The value of the radius may depend on the atom’s state and context.
In this manner, how is atomic radius measured?
Measures of atomic radiusThe radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance. As you can see from the diagrams, the same atom could be found to have a different radius depending on what was around it.
In what unit the radius of an atom is usually expressed?
Angstrom
Don’t Miss: Im.kendall Hunt Answers
Atomic Radius Periodic Table Trends
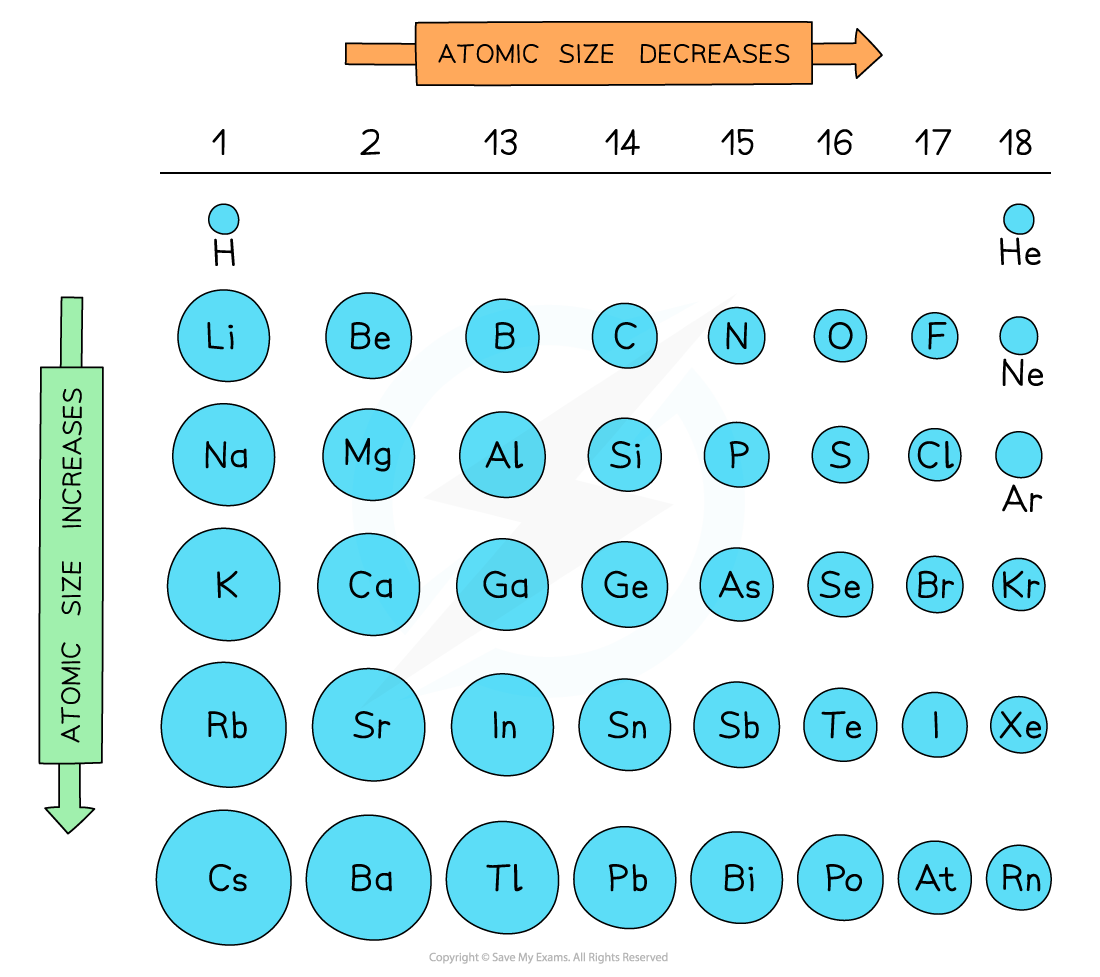
No matter what criteria you use to describe the atomic radius, the size of an atom is dependent on how far out its electrons extend. The atomic radius of an element tends to increase the further down you go in an elementgroup. That’s because the electrons become more tightly packed as you move across the periodic table, so while there are more electrons for elements of increasing atomic number, the atomic radius may decrease. The atomic radius moving down an element period or column tends to increase because an additional electron shell is added for each new row. In general, the largest atoms are at the bottom left side of the periodic table.
How Do You Compare Atomic Radius
How do you compare atomic radius? Atomic radii vary in a predictable way across the periodic table. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Thus, helium is the smallest element, and francium is the largest.
How do atomic radius compare to ions? A comparison of ionic radii with atomic radii shows that a cation, having lost an electron, is always smaller than its parent neutral atom, and an anion, having gained an electron, is always larger than the parent neutral atom.
What determines atomic radius? Summary. Atomic radius is determined as the distance between the nuclei of two identical atoms bonded together. The atomic radius of atoms generally decreases from left to right across a period. The atomic radius of atoms generally increases from top to bottom within a group.
Is atomic size and atomic radius same? What is Atomic Size? Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell. In basic chemistry, the atomic radius is defined as the shortest distance between the atoms nuclei and the outermost shell of the atom.
Don’t Miss: Does Quantum Mechanics Prove God
What Is The Atomic Radius Of Chlorine
I’m trying to study for chemistry and there’s a question in my book about identifying an element based on its atomic radius. But when I looked up the value for chlorine, I found different answers from different sources:
- 1$\begingroup$According to the environmentalchemistry website, the unit is angstroms $\endgroup$
There is no such thing as unified atomic radius. An atomic radius is a class consisting of van der Waals radii $R_\mathrm$ , covalent radii $R_\mathrm$, and ionic radii $R_\mathrm$ .
From the recent edition of CRC Handbook :
$$\begin\hline\text & \text & R_\mathrm~ & R_\mathrm~\\\hline\text & \ce & 1.75 & 1.00\\\hline\end$$
Ionic radii are given for the crystallographic data separately :
$$\begin\hline\text & \text & R_\mathrm~\\\hline\ce} & 3~\text & 0.12\\\ce} & 4 & 0.08\\\hline\end$$
What Are Atomic Radii Or Atomic Radius
Atomic radius or Atomic Radii is the total distance from the nucleus of an atom to the outermost orbital of its electron.
We define the atomic radius of a chemical element as:
The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons.
Atomic radius is similar to the radius of a circle. The nucleus is analogous to be the centre of the circle and the outermost orbital of the electron to the outer edge of the circle. It is difficult to determine the atomic radii because of the uncertainty in the position of the outermost electron.
We use Heisenbergs Uncertainty Principle to obtain a precise measurement of the radius. As per the principle, we determine the radius based on the distance between the nuclei of two bonded atoms. An atom will have different radii depending on the bond it forms, so there is no fixed radius of an atom. The radii of atoms are therefore determined by the bonds they form.
Read Also: Define Composition In Math
Van Der Waals Forces And Atomic Radius
To understand Van der Waals forces and atomic radius rule, you must first understand how the forces in atoms affect their polarity. The first thing you should know is that there are two types of atoms polar atoms and nonpolar atoms.
Every atom contains a nucleus comprised of protons and neutrons. Because the neutrons have no charge of their own to contribute, the nucleus of an atom always contains a positive charge. Outside the nucleus, there is at least one electron shell that holds a certain number of electrons. These electrons are constantly moving. It is important to note, however, that these electrons are not always evenly dispersed.
Atoms with evenly distributed electrons are nonpolar. These nonpolar atoms are the most heavily affected by Van der Waals forces . However, because electrons are constantly moving, it is not likely that a nonpolar bond will last long. Eventually, the number of electrons will be greater on one side of the atom than the other side. When this happens, the atom becomes distorted.
When an atom becomes distorted , it interacts with atoms differently than nonpolarized atoms do. Because there are more protons on one side and more electrons on another, one side is more positive and one side is more negative. For example, if there are more electrons on the left side of an atom compared to the right side of the atom, the left side will be negative and the right side will be positive. This make the atom a dipole.
Covalent Radius Vs Atomic Radius
There are other methods of gauging the size of atoms. Technically, they are all estimates of the atomic radius. However, data tables of the atomic radius are for the distance between the centers of the nuclei of atoms that are just touching each other. In this context, “touching” means the outermost electron shells are in contact with each other. The ionic radius is another method of estimating atom size. The ionic radius is half the distance between two atoms touching each other in a crystal lattice .
The covalent radius and ionic radius may be larger or smaller than the atomic radius of an atom of an element. In general, atomic radius follows a trend in the periodic table in which the radius increases moving down an element group and decreases moving left to right across a period.
Recommended Reading: Go.hrw Algebra 1
Electron Shielding And Atomic Radius
The number of electrons in the inner shells of an atom versus the number of electrons in the outermost shell of the atom will always be in constant competition with each other. The outermost electrons have a negative charge but are attracted to the nucleus of an atom which has positively charged protons. Unfortunately, the inner shell electrons have the same charge, which makes them want to repel the electrons in the outer shell.
At the same time, the protons in the center of the atom are fighting to pull away from each other. Because they all have the same charge, they want to be able to push closer to the electrons than themselves. They are balanced out by the neutrons in the core because neutrons do not have a charge of their own. These neutrons are, however, able to take half the charge from a proton which helps the protons to remain balanced enough to remain in the nucleus. The more powerful a nucleus is, the smaller the atomic radius is because of the strong attraction to the electrons.
What Are The Atomic Radius Trends What Causes Them
There are two main atomic radius trends. One atomic radius trend occurs as you move left to right across the periodic table , and the other trend occurs when you move from the top of the periodic table down . Below is a periodic table with arrows showing how atomic radii change to help you understand and visualize each atomic radius trend. At the end of this section is a chart with the estimated empirical atomic radius for each element.
Read Also: Core Connections Algebra Chapter 1 Answers
What Is The Atomic Radius
Lets discuss the definition of the atomic radius, also called atomic size, and the atomic radius trend on the periodic table. The atomic radius is measured as half the distance between two nuclei of the same atoms that are bonded together. While your initial thought may have been to measure the distance from the center of an atoms nucleus to the edge of its electron cloud, this is inaccurate and not feasible. This is because the borders of orbitals are quite fuzzy, and they also change under different conditions. Thus the atomic radius is measured as shown in the diagram below.
What Is Ionic Radius In Chemistry
4.3/5ionic radiusionionsionic radius
Subsequently, one may also ask, what is ionic radius trend?
The ionic radius trend refers to how the ionic radius of elements follows a predictable trend across the periodic table of the elements. Ionic radius tends to increase as you move from top to bottom down the periodic table, and it tends to decrease as you move left to right across the periodic table.
Furthermore, what is ionic size in chemistry? The ionic size is when the atom loses or gains electrons to become negatively charged or positively charged ions. When atoms lose or gain electrons, the size of the ion is not the same as the original atom.
Herein, what is ionic radius vs atomic radius?
The atomic radius is half the diameter of a neutral atom. In other words, it is half the diameter of an atom, measuring across the outer stable electrons. The ionic radius is half the distance between two gas atoms that are just touching each other.
What is ionic radius measured in?
Ionic radii are typically given in units of either picometers or Angstroms , with 1 Å = 100 pm.
You May Like: Who Is Paris Jacksons Mom
Atomic Radius Versus Ionic Radius
The atomic and ionic radius is the same for atoms of neutral elements, such as argon, krypton, and neon. However, many atoms of elements are more stable than atomic ions. If the atom loses its outermost electron, it becomes a cation or positively charged ion. Examples include K+ and Na+. Some atoms might lose multiple outer electrons, such as Ca2+. When electrons are removed from an atom, it might lose its outermost electron shell, making the ionic radius smaller than the atomic radius.
In contrast, some atoms are more stable if they gain one or more electrons, forming an anion or negatively charged atomic ion. Examples include Cl- and F-. Because another electron shell isn’t added, the size difference between the atomic radius and ionic radius of an anion isn’t as much as for a cation. The anion ionic radius is the same as or slightly larger than the atomic radius.
Overall, the trend for the ionic radius is the same as for the atomic radius: increasing in size moving across and decreasing moving down the periodic table. However, it’s tricky to measure the ionic radius, not the least because charged atomic ions repel each other.