Molecular Shapes And Polarity
Molecules have shapes. There is an abundance of experimental evidence to that effectfrom their physical properties to their chemical reactivity. Small moleculesmolecules with a single central atomhave shapes that can be easily predicted.
The basic idea in molecular shapes is called valence shell electron pair repulsion . It says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. VSEPR makes a distinction between electron group geometry, which expresses how electron groups are arranged, and molecular geometry, which expresses how the atoms in a molecule are arranged. However, the two geometries are related.
There are two types of electron groups: any type of bondsingle, double, or tripleand lone electron pairs. When applying VSEPR to simple molecules, the first thing to do is to count the number of electron groups around the central atom. Remember that a multiple bond counts as only one electron group.
Any molecule with only two atoms is linear. A molecule whose central atom contains only two electron groups orients those two groups as far apart from each other as possible180° apart. When the two electron groups are 180° apart, the atoms attached to those electron groups are also 180° apart, so the overall molecular shape is linear. Examples include BeH2 and CO2:
Identifying Hybridization In Molecules
Figuring out what the hybridization is in a molecule seems like it would be a difficult process but in actuality is quite simple. Because hybridiztion is used to make atomic overlaps, knowledge of the number and types of overlaps an atom makes allows us to determine the degree of hybridization it has. In other words, you only have to count the number of bonds or lone pairs of electrons around a central atom to determine its hybridization.
The following rules give the hybridization of the central atom: 1 bond to another atom or lone pair; = s 2 bonds to another atom or lone pairs = sp 3 bonds to another atom or lone pairs = sp2 4 bonds to another atom or lone pairs = sp3 5 bonds to another atom or lone pairs = sp3d 6 bonds to another atom or lone pairs = sp3d2
This Video Explains it further:
Practice Example:
+ Determine The Molecular Geometry Of Each Of The Following Moleculesgif
33+ Determine The Molecular Geometry Of Each Of The Following Molecules Gif. Vsepr theory and how to determine the shape of a molecule. Hence, the molecular shape of #so_2# is the six bonding pairs arrange themselves with four equatorial bond pairs and one more pair at each of the polar locations.
You May Like: Ccl4 Valence Electrons
For The Following Molecules And Ions Draw The Lewis Structure Then Determine The Number Of
1. For the following molecules and ions, draw the Lewis structure. Then, determine the number of electron domains, the electron-domain geometries, and the molecular geometries. BeCl2 PF3 Number of electron domains Electron domain geometry: Molecular geometry: BCl Number of electron domains Electron domain geometry: Molecular geometry H2S Number of electron domains Electron domain geometry: Molecular geometry PCIs Number of…
View Determine The Molecular Geometry Of Each Of The Followingmolecules Background
View Determine The Molecular Geometry Of Each Of The Following Molecules Background. However, you can choose not to allow certain types of cookies, which may impact your experience of the site and the services we are able to offer. Write the geometry of each type of hybrid orbital that forms.
Recommended Reading: What Does Abiotic Mean In Biology
Predicting Electron Pair Geometry And Molecular Structure
The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures:
The following examples illustrate the use of VSEPR theory to predict the molecular structure of molecules or ions that have no lone pairs of electrons. In this case, the molecular structure is identical to the electron pair geometry.
Predict The Electron Pair Geometry The Molecular Shape And The Bond Angle For A Carbon
72. Predict the electron pair geometry, the molecular shape, and the bond angle for a carbon tetrabromide molecule, CBra, using VSEPR theory. my VSEPR theory 74. Predict the electron pair geometry, the molecular shape, and the bond angle for a phosphine molecule, PH, using VSEPR theory. ecule even though it has four polar bonds. 78. Apply VSEPR theory to explain why SiF, is a nonpolar molecule even though it has four polar bonds. morin O atom or Oion 26….
Recommended Reading: Lesson 9.5 Geometry Answers
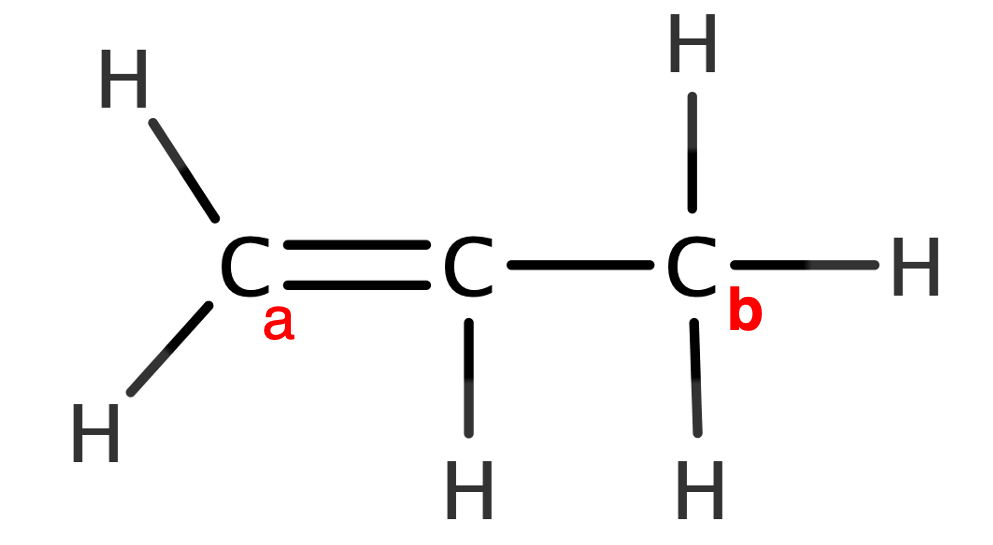
Molecular Geometry Of Acetylene
I need to determine the molecular geometry of acetylene, for this I have performed the following procedure:
First, we represent the correct Lewis structure. Remember that the Lewis structure is a graphical representation that shows the pairs of electrons linking the atoms of a molecule and the pairs of solitary electrons, which may exist.
To predict molecular geometry we use the VSEPR model, which is based on the number of electron pairs in the central atom to determine molecular geometry. In this case, there are two central atoms: C and C’ .
The first carbon, C, has 4 pairs of electrons, all of which are bonding. Using the VSPER notation, $AX_$. For the second carbon, C’, it’s exactly the same. Therefore, molecular geometry is symmetrical and tetrahedral.
For a complete study, we applied the valence bond model based on the hybridization of the atomic orbitals. This is where I don’t know how to see if the link is sigma or pi type. Also, I know that the molecule can be contained in a plane, but I don’t know how to explain it using VSEPR or valence bond theory.
For more detailed info, check “hybridized atomic orbitals”. You will need Molecular Orbital Theory.
This is . In excited state, one electron from 2 s goes to empty 2p, and you will have 1 electron on each 2-shell orbital.
I’ve found a good picture for you, which is below. It explains, why acetylene is linear molecule .
S Used To Find The Shape Of The Molecule
To sum up there are four simple steps to apply the VSEPR theory.
Recommended Reading: Geometry Segment Addition Postulate Worksheet
Is Sf6 Polar Or Nonpolar
SF6 is a nonpolar molecule. This is because the VSEPR theory says that when six fluorine atoms are arranged symmetrically around the sulfur atom, the bond dipoles are cancelled. As a result, it is a nonpolar molecule.
It also has the same properties as non-polar molecules such as being non-soluble in water and being soluble in non-polar organic solvents.
Concluding Remarks
To summarize this article we can say that in the Lewis dot structure of SF6, all the valence electrons are used up which results in forming six single bonds between S-F with no lone pairs of electrons.
The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees.
The molecular geometry of SF6 is octahedral and it is a nonpolar molecule.
About Priyanka
To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Having an MSc degree helps me explain these concepts better. I write all the blogs after thorough research, analysis and review of the topics. And if not writing you will find me reading a book in some cosy cafe!View all posts by Priyanka
Solution For Problem 70p Chapter 10
Introductory Chemistry | 5th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Introductory Chemistry | 5th Edition
PROBLEM 70P
Determine the electron and molecular geometries of each molecule.
;C2H2
;C2H4
; C2H6
Using the geometry of interior atom, the geometry of the molecule will be.
Thus, the electron geometry of the molecule is ;=Tetrahedral and
The molecular geometry of the molecule is = Tetrahedral ;
Also Check: Geometry Segment Addition Postulate Worksheet
Vsepr And Geometry Of Organic Molecules
.for each molecule and then go over each of the following molecular geometries providing lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Vsepr is easy if you follow the right steps. The shape is called octahedral. The molecular structure of the methane molecule, ch4, is shown with a tetrahedral arrangement of build the molecule hcn in the simulator based on the following lewis structure identify the electron pair geometry and the molecular structure of each of the following molecules
Valence Electron Lewis Dol Structure Number Of Domains Electron Orientation Total Bonding Molecular Number Of
Valence Electron Lewis Dol Structure Number of Domains Electron Orientation Total Bonding Molecular Number of Domains Geometry Nonbonding Electrons Hybridization of Central Atom Polarity PCIS Brits XeF. Total Valence Electron Count Lewis Dot Structure Number of Domains Electron Orientation Total Bonding Molecular Number of Domains Geometry Nonbonding Electrons Hybridization of Central Atom Polarity CO Experiment 10-3 Experiments Data and Results: Molecular models aHU Wu NH SO2 SF6
Recommended Reading: How To Login To Imagine Math
Ch3cl Lewis Structure Molecular Geometry Bond Angle And Hybridization
Chloromethane or Methyl chloride having a molecular formula of CH3Cl is an organic compound. It is an odorless and transparent gas that was initially used as a refrigerant. Later it was found that this gas is toxic and can harm the central nervous system of humans. Although it is no longer used as a refrigerant, Chloromethane has many uses and applications in several chemical and pharmaceutical industries.;
To understand its chemical properties and physical properties, one needs first to know the Lewis structure and molecular geometry of CH3Cl. And to help you with understanding its structure in-depth, I will help you to make its Lewis structure step-by-step in this blog post.
But before looking at that, let us first discuss the valence electrons present in this compound as these electrons are the ones that form bonds.
Contents
Molecular Geometry To Determine The Properties Of Molecules
- Slides: 12
Molecular Geometry To determine the properties of molecules it is important to know the ______ molecular ______ geometry. To predict molecular shape chemists developed a theory called ________ which VSEPR stands for: Valence Shell Electron Pair _______ Repulsion __________ VSEPR theory says that valence e- repulsion will cause atoms to arrange themselves as far apart as possible. We will study ____ 5 basic molecular geometries with respect to a central atom. It is necessary to distinguish between ______ bonding pairs of ____ e- and those not shared in a bond. Unbonded or elone _______ pair ____ unpaired electrons are called _______
# of egroups 1 or 2 3 4 4 4 # of lone pairs of e- 0 0 0 1 2 Angle between atoms 180° 120° 109. 5° ~107° ~105° Geometry Trigonal Tetrahedral Trigonal Linear Pyramid Planar Bent
Linear
Trigonal Pyramidal
Bent
Stability e- These valence shells OR All atoms are surrounded by _____. orbitals full of electrons surrounding each clouds OR _______ repel each other because electron clouds are atom _____ negatively ______ charged and like charges repel. Therefore, atoms molecule arrange themselves as ______ far apart in a _______will minimize the repulsion and as possible from one another to _______ make the molecule stable.
Don’t Miss: Introduction To Exponential Functions Common Core Algebra 1 Homework Answer Key
Please Note That Geometry Refers To The Molecular Or Ionic Geometry A What Is The Electron
Please note that “geometry” refers to the molecular or ionic geometry. A. What is the electron-pair geometry for P in PF5? lone pair around the central atom, so the geometry of PF5 is ..There are B. What is the electron-pair geometry for S in SF4? …There are lone pair around the central atom, so the geometry of SF4 is CHECK ANSWER Please note that “geometry” refers to the molecular or ionic geometry. A. What is the electron-pair geometry for CI…
Determine The Correct Molecular Geometry For The
- School
This preview shows page 8 – 13 out of 16 pages.
We have textbook solutions for you!
The document you are viewing contains questions related to this textbook.The document you are viewing contains questions related to this textbook.
Missouri University of Science & Technology
CHEM 1310
Hand on Lab aka Molecular Model and Lewis Structure Lab.docx
Cape Fear Community College
Copy of 3-3 Percent Composition and Formulas Questions.docx
Chesapeake Science Point Charter School
CHEM 121
Missouri University of Science & Technology CHEM 1310
Rec04-Shape&Polarity.pdf
California State University, Fullerton CHEM 120a
Act B6_Molecular Geometry_Procedure_LabReport.docx
St. John’s University CHE 1112R
W3_Moles_Molecular_Formulas_F13
Cape Fear Community College NO 2
Hand on Lab aka Molecular Model and Lewis Structure Lab.docx
Chesapeake Science Point Charter School CHEM 121
Copy of 3-3 Percent Composition and Formulas Questions.docx
We have textbook solutions for you!
The document you are viewing contains questions related to this textbook.The document you are viewing contains questions related to this textbook.
You May Like: Angle Addition Postulate Practice
Molecules With More Than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. We take in account the geometric distribution of the terminal atoms around each central atom. For the final description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the example provided below:
Butane is C4H10. C-C-C-C is the simplified structural formula where the Hydrogens are implied to have single bonds to Carbon. You can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. Let’s start with the leftmost side. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. That means that we have 4 electron groups. By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens.
Electron And Molecular Geometry
For the Electron Geometry, we treat the atoms and electrons equally. The last two molecules in the examples above are both tetrahedral.
SN = 4 atoms + 0 lone pairs = 4
SN = 3 atoms + 1 lone pair = 4
This corresponds to a tetrahedral electron geometry:
However, their molecular geometries are different. For methane , it istetrahedral and for ammonia , it is trigonal pyramidal. The lone pair on the nitrogen is important and if it wasnt there, we would have a hypothetic molecule with a flat/planar geometry:
Why do we ignore the lone pair for naming the molecular geometry? One way to look at it is the fact that electrons are infinitely smaller and lighter than nuclei and when looking on modern microscopes, we dont see them.
Use this table to determine the electron and molecular geometry, for all the combinations of atoms and lone pairs:
Next is a walkthrough of the examples shown in the table following these steps:
1. Draw the Lewis structure for the molecule.
2. Count number of atoms and lone pairs of electrons on the central atom
3. Arrange them in the way that minimizes repulsion .
4. Determine the name of the electron and molecular geometry.
1) Here is the Lewis structure:
2) S.N. = 2 atoms + 0 lone pairs = 2. This falls in the first category in the table and it is an AX2 type.
3) Put the chlorines at 180o
4) This is liner for both the electron and molecular geometry since the Be has no lone pairs.
1) Here is the Lewis structure:
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answer Key
Sf6 Molecular Geometry Lewis Structure Shape And Polarity
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts.
Henri Moissan discovered the existence of SF6. Incidentally, he is also the discoverer of fluorine. The standard way of synthesizing SF6 is to expose S8 to F2. This method causes the formation of a few sulfur fluorides, but those can be eliminated through heating and then using NaOH to remove any additional SF4 molecules.
SF6 cannot be used immediately after synthesis. It needs to be purified to get rid of all reactive fluorides. After that, it needs to go through pyrolysis.;
Here in this blog post, we will learn the Lewis Structure of SF6 and its Bond angles, Molecular geometry and shape that can help us understand the physical properties of this molecule.
| Name of molecule | |
| No of Valence Electrons in the molecule | 48 |