What Does The Vsepr Theory Explain Molecular Shape
The VSEPR theory is used to predict the shape of molecules based on the electron pairs surrounding the central atoms of the molecule. The theory was first put forward in 1940 by Sidgwick and Powell. The VSEPR theory is based on the assumption that the molecule takes a shape such that electron repulsion in the valence shell of that atom is minimized.
Calculate The Number Of Molecular Hybridizations Of The Cs2 Molecule
What is CS2 hybridization? This is a very fundamental question in the field of molecular chemistry. All the molecules are made of atoms. In chemistry, atoms are the fundamental particles. There are four different types of orbitals in chemistry. They are named s, p, d, and f orbitals.
The entire periodic table arrangement is based on these orbital theories. Atoms in the periodic table are classified as follows:
s- block elements
f-block elements
Atoms are classified in the periodic table
CS2 molecule is made of one carbon, two sulfur atoms. The carbon and sulfur atoms have s and p orbitals. But carbon atom has s and p orbitals in the ground state. Carbon comes as the first element in the periodic table of carbon families. The sulfur atom also belongs to the oxygen family group. But it falls as the second element in the periodic table.
When these atoms combine to form the CS2 molecule, its atomic orbitals are mixed and form unique molecular orbitals due to hybridization.
How do you find the CS2 moleculeâs hybridization? We must now determine the molecular hybridization number of CS2.
The formula of CS2 molecular hybridization is as follows:
No. Hyb of CS2= N.A + L.P
No. Hy of CS2 = the number of hybridizations of CS2
Number of C-S bonds = N.A
Lone pair on the central carbon atom = L.P
Calculation for hybridization number for CS2 molecule
No. Hyb of CS2= 2+0=2
Molecular Geometry Notation For Cs2 Molecule :
Determine the form of CS2 molecular geometry using VSEPR theory. The AXN technique is commonly used when the VSEPR theory is used to calculate the shape of the CS2 molecule.
The AXN notation of CS2 molecule is as follows:
The central carbon atom in the CS2 molecule is denoted by the letter A.
The bound pairs of electrons to the core carbon atom are represented by X.
The lone pairs of electrons on the central carbon atom are denoted by the letter N.
Notation for CS2 molecular geometry
We know that carbon is the core atom of CS2, with two electron pairs bound and no lone pairs of electrons. The general molecular geometry formula for CS2 is AX2.
According to the VSEPR theory, if the CS2 molecule has an AX2 generic formula, the molecular geometry and electron geometry will both be linear-shaped forms.
| Name of Molecule | |
| The formal charge of CS2on carbon | 0 |
Recommended Reading: Ccl4 Dot Structure
What Are The Different Types Of Molecular Geometry Shapes
Molecular geometry. The VSEPR theory describes five basic shapes of individual molecules: linear, planar trigonal, tetrahedral, bipyramidal trigonal and octahedral. Apply the VSEPR model to define the geometry of molecules in which the central atom contains one or more pairs of lone electrons. How do you know the shape of the molecules?
Predict The Geometry And Polarity Of The Cs2 Molecule 90+ Pages Explanation Doc
RELATED
79+ pages predict the geometry and polarity of the cs2 molecule 2.2mb. Then click the check boxes at the bottom and right of the simulator to check your answers. Predict The Molecular Geometry And Polarity Of The CS2 Molecule. View Predict The Molecular Geometry And Polarity Of The Cs2 Molecule PNG. Check also: predict and learn more manual guide in predict the geometry and polarity of the cs2 molecule Rotate the molecule to observe the complete geometry.
This problem has been solved. For the detailed reasons of the polarity of CS2 you can also refer to the polarity of CS2.
Co2 Molecular Geometry Science Education And Tutorials
| Title: Co2 Molecular Geometry Science Education And Tutorials |
| Format: eBook |
| Number of Pages: 233 pages Predict The Geometry And Polarity Of The Cs2 Molecule |
| Publication Date: May 2018 |
| Read Co2 Molecular Geometry Science Education And Tutorials |
Also Check: Unit Test Edgenuity Algebra 2
Lewis Structure Of Cs2
If you are a student of chemistry, it is almost obvious that you are aware of the term Lewis Structure. If not, heres a brief explanation of the above-mentioned topic.
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure.
It depends on the octet rule concept and is an extension of the electron dot diagram.
Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
Step 1: The very first step towards drawing the structure of a molecule is to decipher the total number of valence electrons.
A very important point to be noted here is the role of the signs + and -. While the positive + sign indicates the loss of electrons i.e loss of negative charge, the negative – sign is to denote the gain of electrons.
Step 2: The second step is based on finding the central atom of the molecule.
Usually, the one with the highest valence i.e. bonding sites is the central atom.
We can determine the electronegativity value by browsing through the periodic table. As per the trend, it is likely to decrease down a group.
Step 3: Now, we need to draw a skeleton diagram having the presence of single bonds.
Step 4: Next, our task is to complete the octet of the atoms around each of the outer ones with the remaining electrons.
The advisable decision is to finish the electronegative ones first before starting with the electropositive atoms.
How Does Vsepr Determine The Shape Of A Molecule
Use the VSEPR form to define the angles between pairs of links. The repulsion between pairs of valence electrons in the outermost shell of the central atom determines the shape of the molecule. You need to determine the steric number – the number of bonding pairs and lone pairs around the central atom.
Don’t Miss: Punchline Bridge To Algebra Answer Key Page 115
Lewis Structure And Geometrical Structure Of Cs2
Lewis structure of a molecule is also known as electron dot structure because it represents the number of valence electrons of the molecule that take participation in the bond formation.
In lewis structure, the lines denote the bond formed in the molecule and dot represents the non bonded electrons.
Lewis structure is dependent on the octet rule. Octet rule concepts mean that the atom should have eight electrons in its outermost shell to achieve its stability.
In CS2 molecule, Carbon has 4 valence electrons and the Sulfur atom has 6 valence electrons. This makes a total of 16 valence electrons of the CS2 molecule.
Carbon is the least electronegative atom in the molecule therefore it becomes the central atom.
Both Sulfur atoms form a double bond with the carbon bond to complete their octet leaving behind two lone pairs on both Sulfur atom.
And the geometrical shape of the molecule becomes linear having sulfur atoms at both ends. The bond length of the C-S bond is around 155.26 pm.
Below is the image of the geometrical shape of the CS2 molecule.
What Is The Molecular Geometry Of H: 2s
H2S molecular geometry. The hybridization of this H2S molecule is sp3, the sulfur atom is in the middle of the bond with two hydrogen atoms forming a bond angle of less than 180 degrees. According to the VSEPR theory, isolated electron pairs repel each other, but since the sulfur atom is less electronegative, the bond angle decreases by several degrees. This reduction in angle results in a curved design. Another way to learn more about the geometry of molecules is the AXN method.
Don’t Miss: Movement In Geography Examples
Is Cs2 Polar Or Nonpolar
Carbon disulfide is a chemical compound with chemical formula CS2. It is a colorless liquid in appearance and is volatile in nature. It has a sweet odor like that of ether. Many students may also have doubts regarding whether CS2 is polar or not. In this article, I will answer this and will cover its properties and uses.
So, is CS2 polar or nonpolar? CS2 is nonpolar because of its symmetric shape. Although carbon and sulfur differ in their electronegativity and C-S bond is polar, the polarity of both opposite C-S bonds gets canceled by each other resulting in a nonpolar molecule.
Carbon disulfide exists in the liquid state at standard conditions of temperature and pressure. It smells sweet like ether.
This compound is considered as the building block in the world of organic chemistry. It is widely used in the industries as a non-polar solvent.
It is a highly flammable liquid. Its combustion produces SO2 and CO2 gas fumes.
CS2 + 3O2 -combustion-> CO2 + 2SO2
The molecular mass of this compound is 76.13 g·mol1. It is calculated as
Mol mass of CS2 = 1 * 12 + 2 * 32 = 76.13 g·mol1.
The chemical composition of this substance is such that it consists of 1 carbon and 2 sulfur atoms.
The carbon atom is the central atom surrounded by 2 sulfur atoms on both sides. The Sulfur atoms around both sides create the shape of a molecule is linear.
The electronegativity of carbon is 2.55 and that of Sulfur is 2.58 due to which the C-S bonds become slightly polar.
Difference Between Polar And Nonpolar Molecules
The molecules that are held by the covalent bonds can be polar. Let us understand the covalent bonds.
Covalent bonds are the types of chemical bonds in which atoms share electrons of each other to get stabilized.
These bonds can be single, double, and triple depending upon the basis of the number of electrons participated in bond.
Non-Polar Molecules: The non-polar molecules are the molecules in which the charges are uniformly spread across the molecule such that no atom share unequal charge.
The dipole moment of such molecules has zero value. The covalent bond formed between two atoms is non-polar if the atoms have equal electronegativity.
These molecules may also consist of polar bonds within them, but due to the symmetrical shape, the polarity of such bonds gets canceled by each other making the molecule a nonpolar.
Some of the examples of these molecules are BF3, Cl2, O3. You can check out the reason for the non-polarity of C2H4.
Polar Molecules: The nonpolar molecules are the molecules that have unequal distribution of charge across its atoms.
The dipole moment of such molecules is always non zero. The bond formed by a diatomic molecule is said to be polar if they differ in their electronegativity.
This is because higher electronegative atom pulls the bonded electronegative charge slightly towards it and gains a partial negative charge.
Few examples of nonpolar molecules are HCl, HBr, etc. You can check out the reason for the polarity of SCN.
Don’t Miss: What Are 4 Goals Of Psychology
What Is The Molecular Geometry Of The Cs2 Molecule
Linear.
Explanation:
The best place to start when trying to figure out a molecule’s geometry is its Lewis structure.
Carbon disulfide, , will have a total of #16# from the carbon atom and #6# from each of the two sulfur atoms.
The central carbon atom will form double bonds with the two sulfur atoms. These bonds will account for #8# valence electrons of the molecule.
The remaining #8# valence electrons will be placed as lone pairs, two on each sulfur atom.
Now, molecular geometry is determined by the hybridization of the central atom. In this case, the carbon atom is surrounded by two regions of electron density, one for each double bond it forms with the sulfur atoms.
This means that its steric number will be equal to #2# . The carbon atom will thus be #”sp”# hybridized. It will use one s and one p orbitals to form the hybrids, and the remaining p-orbitals to form pi bonds with the two sulfur atoms.
The molecular geometry will thus be linear, the basic #”AX”_2#
How Does Vsepr Determine Molecular Geometry
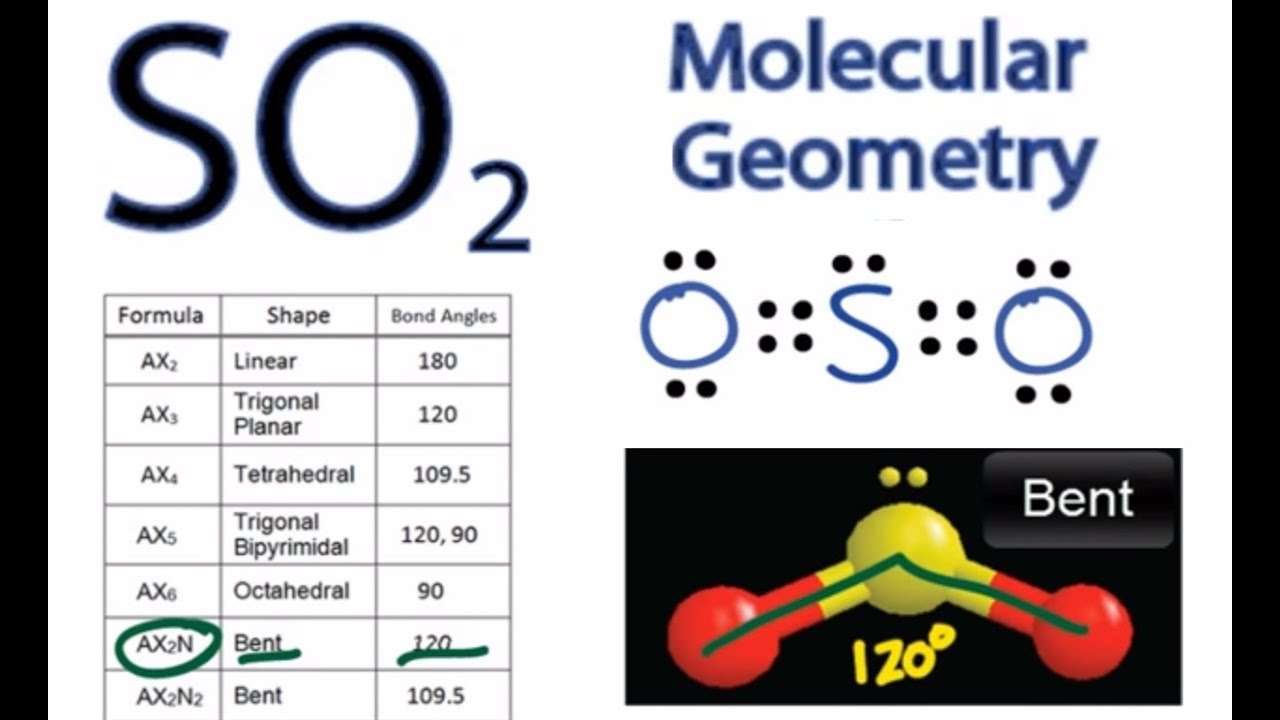
VSEPR uses the steric number and the X and Es distribution to predict the geometric shapes of molecules. AX method: A represents the central atom, X represents the number of sigma bonds between the central atoms and the outer atoms, and E represents the number of lone pairs surrounding the central atom.
Also Check: Simplifying Radicals Worksheet 2
What Is The Molecular Structure Of No2
#2 is a strange electronic molecule, paramagnetic in nature. There are two covalent bonds between N and O in one of the resonance structures of NO2. There is also a coordination bond between N and another odd oxygen electron, which can be in N or O depending on the nature of the resonance structure.
Calculating Lone Pair Of Electrons On The Terminal Sulfur In The Cs2 Geometry:
Finding lone pair of electrons for the terminal sulfur atom is not similar to the central carbon atom. We use the following formula as given below
Use the formula below to find the lone pair on the sulfur atom of the CS2 molecule.
L.P = V.E â N.A
Lone pair on the terminal sulfur atom in CS2 = L.P
Terminal sulfur atomâs valence electron in CS2 = V.E
Number of C-S bonds = N.A
calculation for sulfur atom lone pair in CS2 molecule.
For instance of CS2, their terminal atoms, sulfur, have six electrons in their outermost valence shell, one C-S double bond connection. This gives a total of two C-S double bond connections. But we are considering only one connection for the calculation.
As a result of this, L.P = =4
The lone pair of electrons in the sulfur atom of the CS2 molecule is four. Two sulfur atoms are connected with the central carbon atom.
In the CS2 electron geometry structure, the lone pairs on the central carbon atom are zero, lone pairs of electrons in the sulfur atom have two pairs. Two sulfur atoms have two lone pairs of electrons.
It means there are two lone pairs of electrons in the core carbon atom. No lone pair of electrons on the central carbon atom is responsible for the linear nature of CS2 molecular geometry. But in the structure sulfur atoms are polarised sidewise in their linear geometry.
But in the central, carbon atom has no lone pairs of electrons and two C-S bond pairs stay oppose to each other around 180 degrees.
You May Like: Unit 1 Test Study Guide Geometry Basics
What Geometric Shapes Are The Most Common
Geometric shapes in graphic design meaning squares and rectangles. Squares and rectangles are the most common shapes in graphic projects and can be seen very well on road signs in everyday life. Triangular shapes in graphic design. Meaning of circles, ovals and ellipses. Pentagons, hexagons and octagons in design. Spirals in graphic design.
How Many Molecules Are In H2o
It turns out that a drop of water contains more than a trillion molecules and more than 5 sextillion atoms per drop. To calculate the number of molecules and atoms in a drop of water, you need to know the chemical formula of water. Each water molecule has two hydrogen atoms and one oxygen atom, giving the formula H 2 O.
Don’t Miss: How To Convert In Chemistry
Key Points To Consider When Drawing The Cs2 Molecular Geometry
A three-step approach for drawing the CS2 molecular can be used. The first step is to sketch the molecular geometry of the CS2 molecule, to calculate the lone pairs of the electrons in the central carbon and terminal sulfur atoms the second step is to calculate the CS2 hybridization, and the third step is to give perfect notation for the CS2 molecular geometry.
The CS2 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the CS2 molecule in a specific geometric manner. The geometry of the CS2 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory and molecular hybridization theory, which states that molecules will choose the CS2 geometrical shape in which the electrons have from one another in the specific molecular structure.
Finally, you must add their bond polarities characteristics to compute the strength of the two C-S double bonds . Two sulfur-carbon double bonds in the carbon disulfide, for example, are polarised toward the slightly high electronegative value sulfur atoms, and because all two double bonds have the same size and polarity, their sum is zero due to the CS2 moleculeâs bond dipole moment due to pulling the electron cloud to the two side of linear geometry, and the CS2 molecule is classified as a nonpolar molecule.
Why Is Cs2 A Nonpolar Molecule
As discussed the molecule of carbon disulfide consists of 1 carbon and 2 sulfur atoms on its both sides and forms a symmetric linear-shaped molecule.
The symmetrically shaped molecules are usually nonpolar. Similarly, the CS2 molecule is non-polar because of following reason,
There exists a small difference between the electronegativity of carbon and sulfur atoms that makes the C-S a slightly polar bond.
Both C-S bonds have dipoles in opposite directions as a result it cancels each other and net dipole turns out to be zero.
Therefore, it is generally observed that symmetrically shaped molecules tend to be nonpolar in nature.
Instead of having polar bonds, the molecule is nonpolar.
You May Like: How Did Geography Affect Ancient Greece
Get The Detailed Answer
Use VSEPR theory to decide which one of the following molecules and ions will definitely have at least one 90 bond angle in it. Predict the shapes of molecules and polyatomic ions using vsepr theory. The molecular geometry and polarity of carbon disulfide cs2 using vsepr rules. Carbon disulfide eqrm CS_2 eq is a covalent compound that was first synthesized in 1796 by Wilhelm. Bent nonpolar 1 See answer. Predict the geometry and polarity of the molecule.
Scl2 Molecular Geometry Science Education And Tutorials
| Title: Scl2 Molecular Geometry Science Education And Tutorials |
| Format: ePub Book |
| Number of Pages: 186 pages Predict The Geometry And Polarity Of The Cs2 Molecule |
| Publication Date: |
Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Polar
| Title: Is Ch3oh Polar Or Nonpolar Methanol In 2021 Functional Group Molecules Polar |
| Format: PDF |
| Number of Pages: 274 pages Predict The Geometry And Polarity Of The Cs2 Molecule |
| Publication Date: |
15 Predict The Molecular Geometry And Polarity Of Chegg
| Title: 15 Predict The Molecular Geometry And Polarity Of Chegg |
| Format: PDF |
| Number of Pages: 175 pages Predict The Geometry And Polarity Of The Cs2 Molecule |
| Publication Date: |
| Read 15 Predict The Molecular Geometry And Polarity Of Chegg |
Molecules Free Full Text Calculation Of The Isobaric Heat Capacities Of The Liquid And Solid Phase Of Anic Pounds At 298 15k Means Of The Group Additivity Method Html
Is Cs2 Polar Or Nonpolar
Let’s share :