Physical Properties Of Oxygen
- The gas is colourless, odourless and insipid in a normal state. Liquid oxygen is slightly paramagnetic. It is reactive and forms oxides with every element except helium, neon, krypton, and argon. It is moderately soluble in water.
- Dioxygen is one of the common allotropes of oxygen.
- Trioxygen is the most reactive allotrope of oxygen that would cause damage to lung tissue. This allotrope is termed as ozone.
Polymeric Vs Monomeric Molecular Structures
Oxides of most metals adopt polymeric structures with M-O-M crosslinks. Because these crosslinks are strong, the solids tend to be insoluble in solvents, though they are attacked by acids and bases. The formulas are often deceptively simple. Many are nonstoichiometric compounds. In these oxides, the coordination number of the oxide ligand is 2 for most electronegative elements, and 36 for most metals.
Silicon Dioxide: Silicon dioxide is one of the most common oxides on the surface of earth. Like most oxides, it adopts a polymeric structure.
Although most metal oxides are polymeric, some oxides are monomeric molecules. The most famous molecular oxides are carbon dioxide and carbon monoxide. Phosphorus pentoxide is a more complex molecular oxide with a deceptive name, the formula being P4O10. Some polymeric oxides depolymerize to give molecules when heated. Tetroxides are rare, and there are only five known examples: ruthenium tetroxide, osmium tetroxide, hassium tetroxide, iridium tetroxide, and xenon tetroxide. Many oxyanions are known, such as polyphosphates and polyoxometalates. Oxycations are rarer, an example being nitrosonium . Of course many compounds are known with both oxides and other groups. For the transition metals, many oxo-complexes are known, as well as oxyhalides.
Whats The Difference Between The Carbon Cycle And The Oxygen Cycle
They work independently but are dependent on each other since the carbon cycle releases oxygen for the usage of the oxygen cycle, and the oxygen cycle emits carbon dioxide that goes back to the carbon cycle in turn. The key vehicle in which the processes of oxygen and carbon are related is plants.
To learn more about the different cycles of the planet and more, register with BYJUS and download our app.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: Mcdougal Littell Geometry 9.5 Answers
What Is The Oxygen Cycle In Simple Terms
The cycle of oxygen, the distribution of oxygen through nature in different ways. Free in the air and dissolved in water, in excess of uncombined elements in the atmosphere, oxygen is second only to nitrogen. In order to respire, plants and animals use oxygen and return it as carbon dioxide to the air and water.
What Is The Color Of Oxygen: Properties And Exciting Facts

What is the color of oxygen? Why we, including all forms of life, cannot live without it? What is so special about this element? You may have asked these questions yourself and so have many others.
Indeed, oxygen is one of the most abundant chemical elements on the planet, and it has been baffling scientists since its official discovery in 1773 by Carl Wilhelm Scheele and Joseph Priestley, independently. You will know why I said official when we get to the some facts about oxygen later.
Associated with the chalcogen group, molecular oxygen, dioxygen, or O2, is an extremely volatile covalent compound.
As obvious as it may seem, the discovery of oxygen was key for the development of chemical science: In fact, the process of abstracting electrons from a molecule, known as the chemical process of oxidation, takes its name from this element. This is due to the fact that elemental oxygen has the capacity of forming oxides with most chemical elements.
Technically, it is also the third most abundant element in the universe, trailing behind hydrogen and helium, respectively.;
Hence, in this article, you will learn several facts about this fascinating chemical element. We want to get into its photochemical properties . But also, you will hopefully discover new things to add up to your knowledge. Lets get started.;
Also Check: What Does Competition Mean In Biology
Why Do We Turn Blue When Blood O2 Decreases
Bright red is the color of oxygenated blood because of the protein, hemoglobin. However, when a person experiences hypoxia, hemoglobin will not bind with the red blood cells, resulting in a darker hue, making us appear as bluish.
Basically, oxygen forms a coordination complex with the heme group on hemoglobin. This complex is red-colored, whereas free hemoglobin is actually blue.
General Chemistry Of Oxygen
- Oxides: O-2 ,
- peroxides: O2-2 ,
- super oxide: O2-1.
Oxygen does not react with itself, nitrogen, or water under normal conditions. Oxygen does, however, dissolve in water at 20 degrees Celsius and 1 atmosphere. Oxygen also does not normally react with bases or acids. Group 1 metals are very reactive with oxygen and must be stored away from oxygen in order to prevent them from becoming oxidized. The metals at the bottom of the group are more reactive than those at the top. The reactions of a few of these metals are explored in more detail below.
Lithium: Reacts with oxygen to form white lithium oxide in the reaction below.
Sodium: Reacts with oxygen to form a white mixture of sodium oxide and sodium peroxide. The reactions are shown below.
- oxygen with sodium oxide: \
- oxygen with sodium peroxide: \
Potassium: Reacts with oxygen to form a mixture of potassium peroxide and potassium superoxide. The reactions are shown below.
- Potassium peroxide: \
- potassium superoxide: \
Rubidium and Caesium: Both metals react to produce superoxides through the same process as that of the potassium superoxide reaction.
The oxides of these metals form metal hydroxides when reacting with water. These metal hydroxides make the solution basic or alkaline hence the name alkaline metals.
Group 2 metals react with oxygen through the process of burning to form metal oxides but there are a few exceptions.
Group 15 elements react with oxygen to form oxides. The most important are listed below.
You May Like: What Is Iupac In Chemistry
Magnetic Properties Of Oxygen
As shown, there are two unpaired electrons which causes O2 to be paramagnetic. There are also eight valence electrons in the bonding orbitals and four in antibonding orbitals which makes the bond order 2. This accounts for the double covalent bond that is present in O2.
Video \: A chemical demonstration of the paramagnetism of molecular oxygen, as shown by the attraction of liquid oxygen to magnets.
As shown in Video \, since molecular oxygen has unpaired electrons, it is paramagnetic and is attracted to the magnet. In contrast, molecular nitrogen ) has no unpaired electrons and it not attracted to the magnet.
What Is The Difference Between 2o And O2
Although both the terms 2O and O2 means there are two oxygen atoms, the state of oxygen is different. The key difference between 2O and O2 is that 2O means there are two free oxygen atoms, whereas O2 means it is a molecule having two oxygen atoms. Further, 2O is in the elemental state while O2 is in the molecular state. Importantly, when writing them, we usually write 2 in 2O in the same font size as O, but in O2, we should write the 2 as a subscript of O.
Below info-graphic summarizes the difference between 2O and O2.
You May Like: Holt Mcdougal Pre Algebra Workbook
Properties And Molecular Structure
At standard temperature and pressure, oxygen is a colorless, odorless, and tasteless gas with the molecular formulaO2, referred to as dioxygen.
As dioxygen, two oxygen atoms are chemically bound to each other. The bond can be variously described based on level of theory, but is reasonably and simply described as a covalent double bond that results from the filling of molecular orbitals formed from the atomic orbitals of the individual oxygen atoms, the filling of which results in a bond order of two. More specifically, the double bond is the result of sequential, low-to-high energy, or Aufbau, filling of orbitals, and the resulting cancellation of contributions from the 2s electrons, after sequential filling of the low and * orbitals; overlap of the two atomic 2p orbitals that lie along the O-O molecular axis and overlap of two pairs of atomic 2p orbitals perpendicular to the O-O molecular axis, and then cancellation of contributions from the remaining two of the six 2p electrons after their partial filling of the lowest and * orbitals.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Don’t Miss: Geometry Wars 2 Smile Achievement
General Properties Of Oxygen
First off, we will take a look into molecular oxygens physical and chemical properties.
Oxygen is a colorless and tasteless gas at normal circumstances. This chemical compound is virtually odorless. People have stated, however, that it is actually possible to distinguish between air or pure oxygen. If the odor of oxygen does exist, we may not smell it because of olfactory fatigue.
Os we already mentioned, the most common form of that the element oxygen takes is that of molecular oxygen, dioxygen, or simply O2.
Dioxygen molecules, which are found in gas form under standard conditions, are composed by two atoms of oxygen which are bound through a covalent bond to one another.
However, oxygen is not always in a gas form.
Like most chemical compounds, under certain conditions, which we are about to discuss, it can also transition to different states of matter.
Corrosionpedia Explains Chemical Oxygen Demand
Chemical oxygen demand testing is typically performed using a strong oxidizing chemical. Organic matter is oxidized into carbon dioxide and water in an acidic condition. The quantity of organic matter or the demand of oxygen is calculated by determining how much oxidizing chemical was consumed during the test.
Chemical oxygen demand tests are typically performed on wastewater. The pollution level is calculated by measuring the amount of organic matter in the water. Water with too much organic material can have a negative effect on the environment in which the wastewater is discharged.
Chemical oxygen demand is similar to biochemical oxygen demand in that they are both used to calculate the oxygen demand of a water sample. The difference between the two is that chemical oxygen demand measures everything that can be oxidized, whereas biochemical oxygen demand only measures the oxygen demanded by organisms.
Recommended Reading: How Did Geography And Religion Influence Ethiopia’s Development
Common Uses For Oxygen The Element In Everyday Life
- Post author
Oxygen is an odorless and colorless gas with atomic number 8 on the periodic table. This chemical element is also known as O and along with sulfur, selenium, tellurium and polonium, they fall into the category of chalcogens. The chalcogens is a group of chemical elements that occupy column number 16 and share similar physical and chemical characteristics. Oxygen is the third the most abundant chemical element that can be found in nature. About 20% of the Earths atmosphere is composed of diatomic oxygen or O2. So, here are common uses for oxygen the element in daily life:
Chemical Properties of Oxygen
Swedish-German chemist Carl Wilhelm Scheele was the first person who discovered the existence of oxygen. He tried to analyze the reaction between melted saltpetre and acetic acid which resulted to the production of a red vapor. This discovery then led other scientists to come up with a bunch of combustion theories and further experiments. The term oxygen was first coined by Antoine-Laurent de Lavoisier, a chemist from France, in 1777. Although it was first discovered in the late of 18th century, ancient civilizations and older scientists had conducted various experiments before.
;You may also see:
You may also see:
Life Support And Recreational Use
An application of O2 as a low-pressure breathing gas is in modern space suits, which surround their occupant’s body with the breathing gas. These devices use nearly pure oxygen at about one-third normal pressure, resulting in a normal blood partial pressure of O2. This trade-off of higher oxygen concentration for lower pressure is needed to maintain suit flexibility.
Scuba and surface-suppliedunderwater divers and submariners also rely on artificially delivered O2. Submarines, submersibles and atmospheric diving suits usually operate at normal atmospheric pressure. Breathing air is scrubbed of carbon dioxide by chemical extraction and oxygen is replaced to maintain a constant partial pressure. Ambient pressure divers breathe air or gas mixtures with an oxygen fraction suited to the operating depth. Pure or nearly pure O2 use in diving at pressures higher than atmospheric is usually limited to rebreathers, or at relatively shallow depths , or medical treatment in recompression chambers at pressures up to 2.8 bar, where acute oxygen toxicity can be managed without the risk of drowning. Deeper diving requires significant dilution of O2 with other gases, such as nitrogen or helium, to prevent oxygen toxicity.
Other recreational uses that do not involve breathing include pyrotechnic applications, such as George Goble‘s five-second ignition of barbecue grills.
You May Like: What Is Parasitism In Biology
Where Did Oxygen Originate On Earth
Oxygen comes in third as the most abundant element across the whole universe. However this only accounts for about 1% of oxygen, since the two main constituents, hydrogen and helium, account for 75% and 23% of the entire universe, respectively.
But it was relatively scarce during the formation of Earth.
Accordingly to theories, early forms of cyanobacteria have produced oxygen and added it into the atmosphere of our then-prehistoric planet. Like plants of today, these organisms used photosynthesis as a form of sustenance. For millions of years, they took in carbon dioxide and released oxygen a grand event dubbed as the Great Oxidation Event.;;
Oxygen Exhibits High Reactivity
Due to its electronegativity, oxygen forms stable chemical bonds with almost all elements to give the corresponding oxides. Noble metals are prized because they resist direct chemical combination with oxygen, and substances like gold oxide must be generated by indirect routes. Two independent pathways for corrosion of elements are hydrolysis and oxidation by oxygen. The combination of water and oxygen is even more corrosive. Virtually all elements burn in an atmosphere of oxygen or an oxygen-rich environment. In the presence of water and oxygen , some elementsfor example, sodiumreact rapidly, even dangerously, to give hydroxide products. In part for this reason, alkali and alkaline earth metals are not found in nature in their metallic form. Cesium is so reactive with oxygen that it is used as a getter in vacuum tubes. Solutions of potassium and sodium, are used to deoxygenate and dehydrate some organic solvents.
Read Also: How To Study For Ap Human Geography
Difference Between Oxygen And Ozone
October 12, 2011 Posted by Madhu
The key difference between oxygen and ozone is that the oxygen is a diatomic gaseous molecule of oxygen element, whereas ozone is a triatomic molecule of oxygen.
Oxygen gas and ozone are the most familiar allotropes of oxygen element. Oxygen is an extremely important gas for living organisms; for their respiration. Ozone also protects life on earth when it is in the upper atmosphere, but at the lower atmosphere, it is harmful.
Use In Space Suits And Scuba Diving Suits
A notable application of O2 as a low-pressure breathing gas is in modern space suits, which surround their occupants body with pressurized air. These devices use nearly pure oxygen at about one third normal pressure, resulting in a normal blood partial pressure of O2. This trade-off of higher oxygen concentration for lower pressure is needed to maintain flexible spacesuits.
Scuba divers and submariners also rely on artificially delivered O2, but most often use normal pressure and/or mixtures of oxygen and air. O2 use in diving at higher than sea-level pressures is usually limited to rebreather, decompression, or emergency treatment use at relatively shallow depths . Deeper diving requires significant dilution of O2 with other gases, such as nitrogen or helium, to help prevent oxygen toxicity. People who climb mountains or fly in non-pressurized fixed-wing aircrafts sometimes have supplemental O2 supplies.
You May Like: How To Solve For Time In Physics
What Does Chemical Oxygen Demand Mean
Chemical oxygen demand is the amount of oxygen needed to oxidize the organic matter present in water. Chemical oxygen demand testing is used to determine the amount of oxidation that will occur and the amount of organic matter in a water sample. Chemical oxygen demand testing is also used to determine the amount of inorganic chemicals in a sample.
Isotopes And Stellar Origin
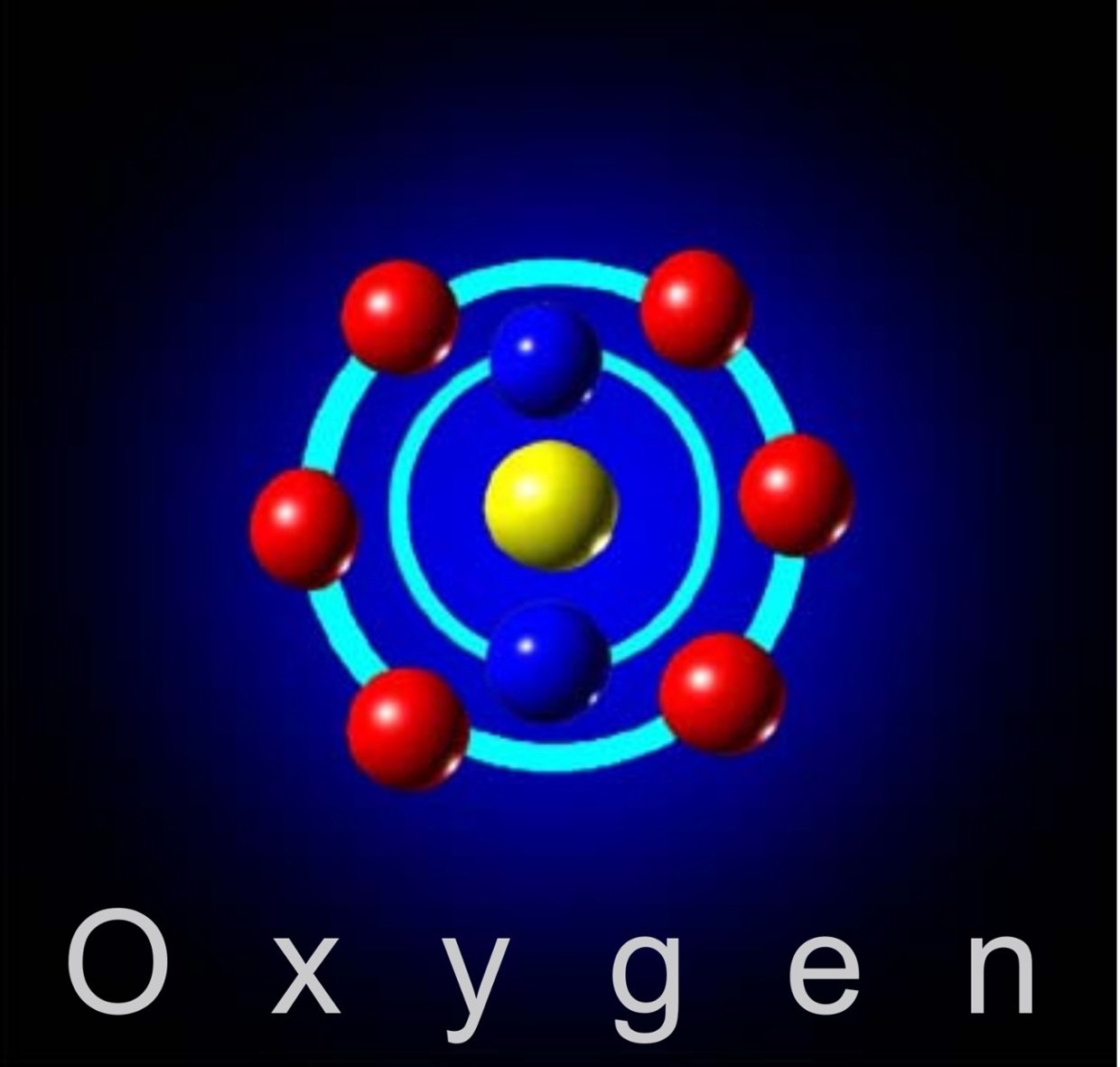
Naturally occurring oxygen is composed of three stable isotopes, 16O, 17O, and 18O, with 16O being the most abundant .
Most 16O is synthesized at the end of the helium fusion process in massive stars but some is made in the neon burning process.17O is primarily made by the burning of hydrogen into helium during the CNO cycle, making it a common isotope in the hydrogen burning zones of stars. Most 18O is produced when 14N captures a 4He nucleus, making 18O common in the helium-rich zones of evolved, massive stars.
Fourteen radioisotopes have been characterized. The most stable are 15O with a half-life of 122.24;seconds and 14O with a half-life of 70.606;seconds. All of the remaining radioactive isotopes have half-lives that are less than 27;s and the majority of these have half-lives that are less than 83;milliseconds. The most common of the isotopes lighter than 16O is + decay to yield nitrogen, and the most common mode for the isotopes heavier than 18O is beta decay to yield fluorine.
Also Check: What Does Abiotic Mean In Biology