How Activation Energy Works In Chemistry
Chemical reactions need a certain amount of energy to begin working. Activation energy is the minimum energy required to cause a reaction to occur.
To understand activation energy, we must first think about how a chemical reaction occurs.
Anyone who has ever lit a fire will have an intuitive understanding of the process, even if they have not connected it to chemistry.
Most of us have a general feel for the heat necessary to start flames. We know that putting a single match to a large log will not be sufficient and a flame thrower would be excessive. We also know that damp or dense materials will require more heat than dry ones. The imprecise amount of energy we know we need to start a fire is representative of the activation energy.
For a reaction to occur, existing bonds must break and new ones form. A reaction will only proceed if the products are more stable than the reactants. In a fire, we convert carbon in the form of wood into CO2 and is a more stable form of carbon than wood, so the reaction proceeds and in the process produces heat. In this example, the activation energy is the initial heat required to get the fire started. Our effort and spent matches are representative of this.
We can think of activation energy as the barrier between the minima of the reactants and products in a chemical reaction.
Temperature Dependence And The Relation To The Arrhenius Equation
The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the rate at which a reaction proceeds. From the equation, the activation energy can be found through the relation
- k ) }}/}}
where A is the pre-exponential factor for the reaction, R is the universal gas constant, T is the absolute temperature , and k is the reaction rate coefficient. Even without knowing A, Ea can be evaluated from the variation in reaction rate coefficients as a function of temperature .
At a more advanced level, the net Arrhenius activation energy term from the Arrhenius equation is best regarded as an experimentally determined parameter that indicates the sensitivity of the reaction rate to temperature. There are two objections to associating this activation energy with the threshold barrier for an elementary reaction. First, it is often unclear as to whether or not reaction does proceed in one step; threshold barriers that are averaged out over all elementary steps have little theoretical value. Second, even if the reaction being studied is elementary, a spectrum of individual collisions contributes to rate constants obtained from bulk experiments involving billions of molecules, with many different reactant collision geometries and angles, different translational and vibrational energiesâall of which may lead to different microscopic reaction rates.
Problem 1 Tutorial: Features Of Enzyme Catalyzed Reactions
Which statement about enzyme catalyzed reactions is NOT true?
A. enzymes form complexes with their substrates. B. enzymes lower the activation energy for chemical reactions. C. enzymes change the Keq for chemical reactions. D. many enzymes change shape slightly when substrate binds. E. reactions occur at the “active site” of enzymes, where a precise 3D orientation of amino acids is an important feature of catalysis.
Features of Enzyme Catalyzed Reactions
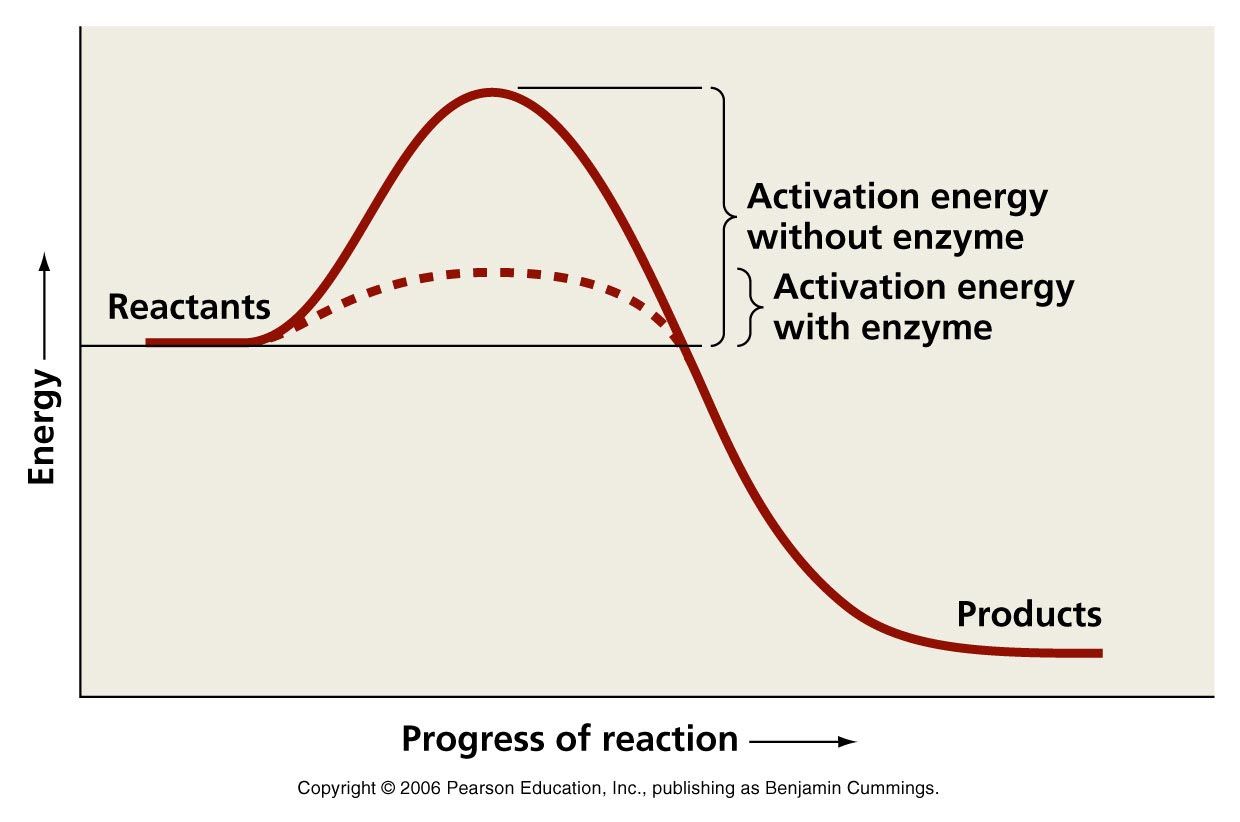
Enzymes are biological catalysts. Catalysts lower the activation energy for reactions. The lower the activation energy for a reaction, the faster the rate. Thus enzymes speed up reactions by lowering activation energy. Many enzymes change shape when substrates bind. This is termed “induced fit”, meaning that the precise orientation of the enzyme required for catalytic activity can be induced by the binding of the substrate.
Enzymes have active sites. The enzyme active site is the location on the enzyme surface where substrates bind, and where the chemical reaction catalyzed by the enzyme occurs. There is a precise substrate interaction that occurs at the active site stabilized by numerous weak interactions .
Enzymes form complexes with their substrates. The binding of a substrate to an enzyme active site is termed the “enzyme-substrate complex.” A generic equation for complex formation is as follows:
You May Like: What Not To Do In The Chemistry Lab
The First Law Of Thermodynamics
The first law of thermodynamics deals with the total amount of energy in the universe. The law states that this total amount of energy is constant. In other words, there has always been, and always will be, exactly the same amount of energy in the universe.
Energy exists in many different forms. According to the first law of thermodynamics, energy can be transferred from place to place or changed between different forms, but it cannot be created or destroyed. The transfers and transformations of energy take place around us all the time. For instance, light bulbs transform electrical energy into light energy, and gas stoves transform chemical energy from natural gas into heat energy. Plants perform one of the most biologically useful transformations of energy on Earth: they convert the energy of sunlight into the chemical energy stored within organic molecules.
The first law of thermodynamics: Shown are two examples of energy being transferred from one system to another and transformed from one form to another. Humans can convert the chemical energy in food, like this ice cream cone, into kinetic energy by riding a bicycle. Plants can convert electromagnetic radiation from the sun into chemical energy.
What Is Activation Energy
Activation energy is one of the essential forces that allows chemical reactions to occur. This energy helps to explain how reactions occur and why it is not common for reactions to occur in nature. It also explores the strength of different elemental bonds and why some elements are more reactive than other elements.
It is known that molecules can be combined or transformed into different products with enough energy. This is because each molecule contains a certain amount of energy sometimes kinetic, sometimes potential. This energy allows chemical reactions to occur. When two molecules collide, the energy inside the molecules has the potential to cause a transition state that turns stable bonds into unstable bonds.
If a collision is powerful enough to disrupt a stable bond, a chemical reaction can occur and create a new product. To ensure that a collision between molecules is strong enough to create a reaction, a certain amount of energy is needed. The minimum amount of energy to create these reactions is known as activation energy.
You May Like: Practice 2 4 Reasoning In Algebra Answer Key
Where Does Activation Energy Come From
Where does the activation energy required by chemical reactants come from? The source of the activation energy needed to push reactions forward is typically heat energy from the surroundings. Heat energy speeds up the motion of molecules, increasing the frequency and force with which they collide. It also moves atoms and bonds within the molecule slightly, helping them reach their transition state.
For this reason, heating up a system will cause chemical reactants within that system to react more frequently. Increasing the pressure on a system has the same effect. Once reactants have absorbed enough heat energy from their surroundings to reach the transition state, the reaction will proceed.
The activation energy of a particular reaction determines the rate at which it will proceed. The higher the activation energy, the slower the chemical reaction will be. The example of iron rusting illustrates an inherently slow reaction. This reaction occurs slowly over time because of its high EA.
Additionally, the burning of many fuels, which is strongly exergonic, will take place at a negligible rate unless their activation energy is overcome by sufficient heat from a spark. Once they begin to burn, however, the chemical reactions release enough heat to continue the burning process, supplying the activation energy for surrounding fuel molecules.
Activation Energy: Why Getting Started Is The Hardest Part
The beginning of any complex or challenging endeavor is always the hardest part. Not all of us wake up and jump out of bed ready for the day. Some of us, like me, need a little extra energy to transition out of sleep and into the day. Once Ive had a cup of coffee, my energy level jumps and Im good for the rest of the day. Chemical reactions work in much the same way. They need their coffee, too. We call this activation energy.
Understanding how this works can be a useful perspective as part of our latticework of mental models.
Whether you use chemistry in your everyday work or have tried your best not to think about it since school, the ideas behind activation energy are simple and useful outside of chemistry. Understanding the principle can, for example, help you get kids to eat their vegetables, motivate yourself and others, and overcome inertia.
Don’t Miss: Lesson 9.5 Geometry Answers
The System And Surroundings
Thermodynamics often divides the universe into two categories: the system and its surroundings. In chemistry, the system almost always refers to a given chemical reaction and the container in which it takes place. The first law of thermodynamics tells us that energy can neither be created nor destroyed, so we know that the energy that is absorbed in an endothermic chemical reaction must have been lost from the surroundings. Conversely, in an exothermic reaction, the heat that is released in the reaction is given off and absorbed by the surroundings. Stated mathematically, we have:
\Delta \text=\Delta \text_}+\Delta \text_}=0
The system and surroundings: A basic diagram showing the fundamental distinction between the system and its surroundings in thermodynamics.
Relationship Between Activation Energy And Gibbs Energy
Activation energy is a term in the Arrhenius equation used to calculate the energy needed to overcome the transition state from reactants to products. The Eyring equation is another relation that describes the rate of reaction, except instead of using activation energy, it includes Gibbs energy of the transition state. The Gibbs energy of the transition state factors in both enthalpy and entropy of a reaction. Activation energy and Gibbs energy are related, but not interchangeable.
You May Like: What Is Energy In Quantum Physics
Endergonic And Exergonic Reactions
If energy is released during a chemical reaction, then the resulting value from the above equation will be a negative number. In other words, reactions that release energy have a G < 0. A negative G also means that the products of the reaction have less free energy than the reactants because they gave off some free energy during the reaction. Reactions that have a negative G and, consequently, release free energy, are called exergonic reactions. Exergonic means energy is exiting the system. These reactions are also referred to as spontaneous reactions because they can occur without the addition of energy into the system. Understanding which chemical reactions are spontaneous and release free energy is extremely useful for biologists because these reactions can be harnessed to perform work inside the cell. An important distinction must be drawn between the term spontaneous and the idea of a chemical reaction that occurs immediately. Contrary to the everyday use of the term, a spontaneous reaction is not one that suddenly or quickly occurs. The rusting of iron is an example of a spontaneous reaction that occurs slowly, little by little, over time.
Exergonic and Endergonic Reactions: Exergonic and endergonic reactions result in changes in Gibbs free energy. Exergonic reactions release energy; endergonic reactions require energy to proceed.
Collisions With Limited Energy
Collisions between molecules that are not given enough activation energy are not able to reach the transition state. This means that while they are receiving some activation energy, they are not able to disrupt the stable bonds that are found with the reactants. Because there is not enough energy applied to the reaction, the molecules are not able to re-arrange and form the desired end-product.
Recommended Reading: What Is Gradualism In Biology
A Brief History Of Activation Energy
The idea of activation energy was first proposed by Svante Arrhenius a Swedish chemist who helped to pioneer the field of physical chemistry. Arrhenius was born in 1859 and quickly proved himself to be a prodigy in mathematics. It was this understanding of arithmetic that helped him to advance quickly in the field of science and make many discoveries in the field of physical chemistry.
He studied chemistry at university and put together a 150 page dissertation with 56 different theses in 1884 to obtain his doctorate. Although the professors in Sweden who reviewed his dissertation did not think highly of his work, almost all 56 theses have been accepted by modern physical chemistry with small changes or no changes at all. His continued studies of the subjects put forth in these theses would eventually lead to his discovery of activation energy.
In 1889, Svante Arrhenius would finally make the discovery of activation energy. By studying chemical reactions and looking at the work of his colleagues, he observed that most reactions needed heat to be successful. This made him curious as to why heat was so necessary to form chemical reactions.
Why Is Activation Energy Needed
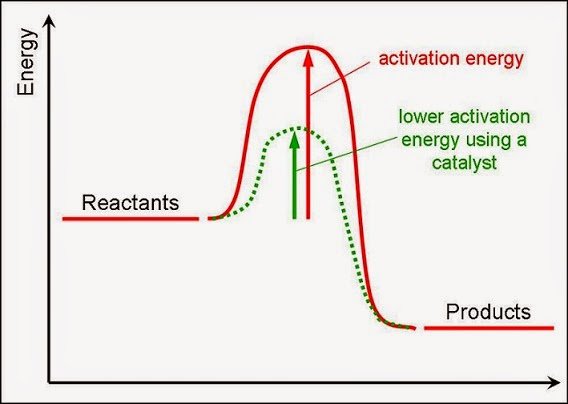
If you mix together two chemicals, only a small number of collisions will naturally occur between the reactant molecules to make products. This is particularly true if the molecules have low kinetic energy. So, before a significant fraction of reactants can be converted into products, the free energy of the system must be overcome. The activation energy gives the reaction that little extra push needed to get going. Even exothermic reactions require activation energy to get started. For example, a stack of wood won’t start burning on its own. A lit match can provide the activation energy to start combustion. Once the chemical reaction starts, the heat released by the reaction provides the activation energy to convert more reactant into product.
Sometimes a chemical reaction proceeds without adding any additional energy. In this case, the activation energy of the reaction is usually supplied by heat from the ambient temperature. Heat increases the motion of the reactant molecules, improving their odds of colliding with each other and increasing the force of the collisions. The combination makes it more likely bonds between reactant will break, allowing for the formation of products.
Don’t Miss: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Enzymes Lower Activation Energy
Enzymes are an important class of proteins that help in cellular processes. Enzymes are particular in their binding and can be allosterically regulated. In enzyme-catalyzed reactions, the enzymes lower the activation energy needed for a certain chemical reaction. The free energy of the reactants and products do not change, just the threshold energy level needed for the reaction to commence. Enzymes can lower the activation energy of a chemical reaction in three ways. One of the ways the activation energy is lowered is having the enzyme bind two of the substrate molecules and orient them in a precise manner to encourage a reaction. This can be thought of as lining the binding pockets up for the substrates so that it is not left to random chance that they will collide and be oriented in this way. Another way enzymes can lower the activation energy by rearranging the electrons in the substrate so that there are areas that carry partial positive and partial negative charges which favor a reaction to occur. Lastly, the enzyme can strain the bound substrate which forces it to a transition state that favors a reaction. By manipulating the substrates of the reaction, the enzyme can lower the necessary energy needed to make the reaction occur. The enzyme itself is not a component of the chemical reaction and is the same molecule at the beginning of the reaction as it is at the end.
Meaning Of Activation Energy
To understand its concept, we can visualize it as the magnitude of the energy barrier i.e. the potential barrier. This barrier separates the minima of potential energy surfaces. It involves the initial and the final thermodynamic state of the system.
To define it, we have to analyze the initiation of chemical reactions. These reactions occur when molecules exchange electrons or when we bring together ions with opposite charges. To exchange electrons in the molecules, the bonds keeping the electrons tied with the molecule, have to be broken.
An external energy source can give the energy which is required to dislodge the electrons and hence can allow the chemical reaction to proceed. We express activation energy in units such as kilojoules, kilocalories or kilowatt-hours.
Once the reaction is on the way, it releases energy and then it is self-sustaining. So, we need this activation energy only at the beginning, to let the chemical reaction start.
Also Check: What Is Mean Median Mode And Range In Math
Chemical Reactions Proceed Through High
All chemical reactions proceed through one or more transition-state intermediates whosecontent of free energy is greater than that of either the reactants or the products. For thesimple reaction R P, we can write
energy; Kis the equilibrium constant for the reactionRS,vGG
Hypothetical energy changes in the conversion of areactantsay, glyceraldehyde 3-phosphateto a productsay,dihydroxyacetone phosphate in
The rate V of the overall reaction R S will be proportional tothe rate constant v and to the number of molecules in the transition state S,that is, the concentration of the transition-state intermediate, :
But since S is in equilibrium with R, the reactant, we can write
KGGVGGG
In some reactions, certain covalent bonds are moved to a strained position in the transitionstate, and an input of energythe energy of activationGisessential for this to happen. In other reactions, formation of the transition state involvesexcitation of electrons, which likewise requires an input of energy; only then can theelectrons pair up, forming a in the product. In still other reactions, moleculesneed only enough energy to overcome the mutual repulsion of their electron clouds to get closeenough to react.
The conversion of glyceraldehyde 3-phosphate to dihydroxyacetone phosphate involves an intermediate. Two groups, a base B and an acid HA, are parts of triosephosphateisomerase, the enzyme that catalyzes this reaction. To form the