Density Of Various Materials Examples
1000 kg/m33.98oC . less dense as it freezes ice floats
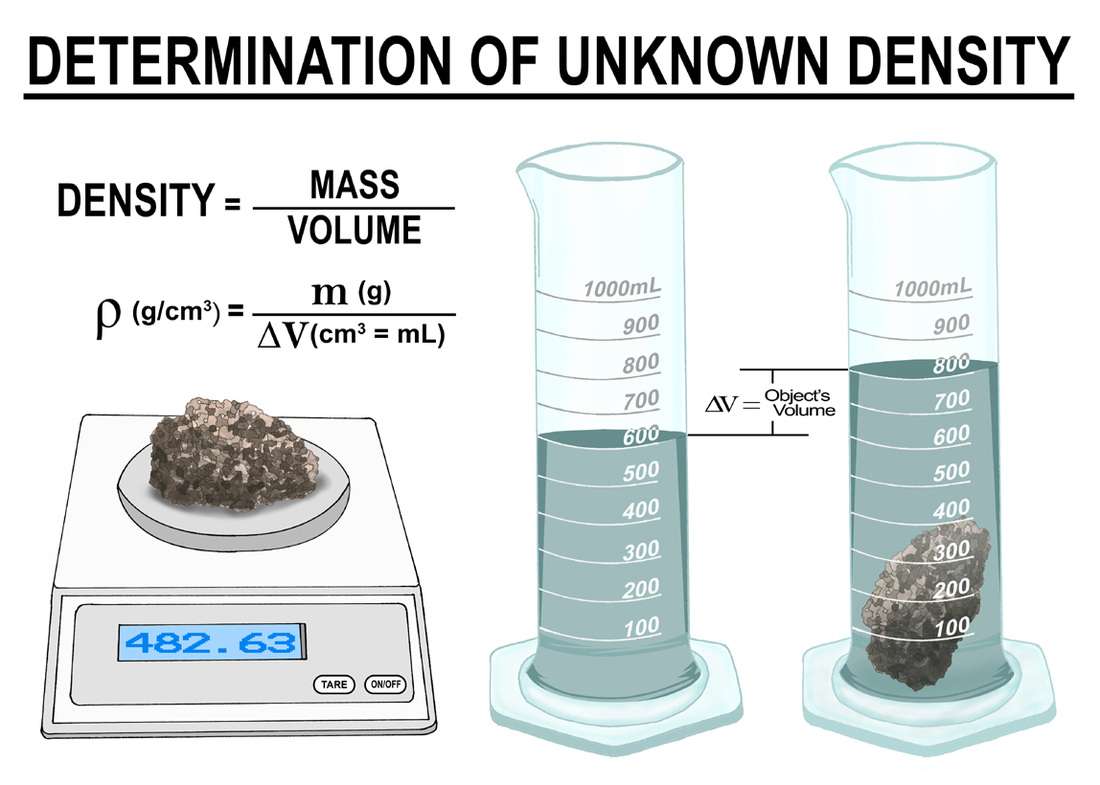
= m/V = 1/
The specific volume of a substance is the total volume of that substance divided by the total mass of that substance . It has units of cubic meter per kilogram .
heavy water11% greater than water
This difference is caused by the fact, the deuterium nucleus is twice as heavy as hydrogen nucleus. Since about 89% of the molecular weight of water comes from the single oxygen atom rather than the two hydrogen atoms, the weight of a heavy water molecule, is not substantially different from that of a normal water molecule. The molar mass of water is M = 18.02 and the molar mass of heavy water is M = 20.03 , therefore heavy water has a density about 11% greater .
Pure heavy water has its highest density 1110 kg/m3 at temperature 3.98oC . Also heavy water differs from most liquids in that it becomes less dense as it freezes. It has a maximum of density at 3.98 °C , whereas the density of its solid form ice is 1017 kg/m3. It must be noted, the change in density is not linear with temperature, because the volumetric thermal expansion coefficient for water is not constant over the temperature range.
well knowndensity
The density of any substance is the reciprocal of its specific volume .
= m/V = 1/
The specific volume of a substance is the total volume of that substance divided by the total mass of that substance . It has units of cubic meter per kilogram .
Density Of Water Experiment
To understand the density of water, lets do a small experiment. We will need a tall glass cup, honey, water, coconut oil and food colouring,
Step 1: Pour a one-quarter cup of honey,
Step 2: Pour a one-quarter cup of coloured water gently on top of the honey.
Step 3: pour a one-quarter cup of coconut oil on top of the coloured water.
Now, you will notice that different substance has a different density, which means for the same volume different substances weigh differently, as they weigh differently heavier substances tend to settle at the bottom, like honey and lighter material like oil tends to float at the top.
Densest Materials On The Earth
Since nucleons make up most of the mass of ordinary atoms, the density of normal matter tends to be limited by how closely we can pack these nucleons and depends on the internal atomic structure of a substance. The densest material found on earth is the metal osmium, but its density pales by comparison to the densities of exotic astronomical objects such as white dwarf stars and neutron stars.
List of densest materials:
It must be noted, plutonium is a man-made isotope and is created from uranium in nuclear reactors. But, In fact, scientists have found trace amounts of naturally-occurring plutonium.
If we include man made elements, the densest so far is Hassium. Hassium is a chemical element with symbol Hs and atomic number 108. It is a synthetic element and radioactive. The most stable known isotope, 269Hs, has a half-life of approximately 9.7 seconds. It has an estimated density of 40.7 x 103 kg/m3. The density of Hassium results from its high atomic weight and from the significant decrease in ionic radii of the elements in the lanthanide series, known as lanthanide and actinide contraction.
Read Also: How To Convert In Chemistry
The Importance Of Density: Buoyancy
Density has obvious importance when it comes to the buoyancy of objects. Broadly, if something is denser than water it will sink, but if something has a lower density than water, it will float.
More technically, something will start to float when the weight of the water it displaces matches the weight of the object, but if this never happens it will sink. If the object is denser than water , the weight of the water it displaces can never match the weight of the object, so it will continue to sink.
Aluminum is a good example. It is denser than water, but a piece of aluminum foil stretched out will float on water because of the large surface area making contact with the water. However, if you roll the same amount of foil up into a ball, the surface area in contact with the water becomes much smaller and the mass is concentrated above it, so the greater density of aluminum wins out and the foil will sink. This is why boats made of denser materials than water will float, even though a single block of the material will sink: The whole structure has a lower density than the block because it contains a lot of air or less dense material too.
Materials For The Class

- Project the image Wood
- Wood is made mostly from carbon, hydrogen, and oxygen atoms bonded together into a molecule called glucose. These glucose molecules are bonded together to form long chains called cellulose. Many cellulose molecules stacked together give wood its structure and density.
In general, the density of wood and plastic are similar because they are made of similar atoms arranged in long chains. The difference in density is mostly based on the arrangement and packing of the polymer chains. Also, since wood is from a living thing, its density is affected by the structure of plant cells and other substances that make up wood.
Ask students:
The size, mass, and arrangement of atoms affect the density of a substance.
- How might these factors work together to cause a substance to have a high density?
- A substance with smaller more massive atoms that are close together is going to have a higher density.
- How might these factors work together to cause a substance to have a low density?
- A substance with larger, lighter atoms that are farther apart is going to have a lower density.
Extend
You May Like: Holt Geometry Chapter 7
Applications Of Density In Real Life
Many applications of density are there in our real-life like a few examples are in pipe design, shipbuilding, helium balloons, weight distribution in the airplane, and the fact that ice floats on water.
- The knowledge of the densities of two substances helps you in separation techniques. For example, separation of oil from water. Leakage of an oil tank in the ocean then oil drops start to float on the water due to their less density in the water.
- Another well-known application of density is determining whether an object will float on water or not. The floating of ships and diving of submarines are due to their density difference.
What Is Energy Density
Energy Density can be defined as the total amount of energy in a system per unit volume. For example, the number of calories available per gram weight of the food. Foodstuffs have low energy density and will provide less energy per gram of the food. It means that we can eat more of them due to fewer calories.
Therefore we may say that energy density is the amount of energy accumulated in a system per unit volume. It is denoted by letter U. Magnetic and electric fields are also the main sources for storing the energy.
You May Like: How Many Biological Children Does Nicole Kidman Have
Applications Of Dft/molecular Simulation To Psd Analysis
DFT and molecular simulation methods have been applied to the analysis of adsorbents in two main capacities. For nonporous adsorbents, DFT can be used to provide a local isotherm in order to solve eq. for the distribution of site energies on the adsorbent surface . A sample result is shown in Fig. 5 for the site energy distribution of a heterogeneous activated carbon obtained from DFT analysis of the nitrogen sorption isotherm.
Figure 5. Surface energy distribution of a heterogeneous nonporous carbon, ASTM C4 nitrogen isotherm of ASTM C4 at 77 K, with fitted DFT result .
John L. Lyons, … Chris G. Van de Walle, in, 2013
The Importance Of Density: Calculating Mass
Since density and mass are so closely related, you can calculate the mass of a certain amount of a substance easily provided you know its density and the volume of the substance. This can be useful in engineering and other applications. Use the simple formula:
To work out the mass of the substance. For example, using the density of steel quoted earlier, 0.5 cubic meters of steel has a mass of:
This is useful in many different situations. For example, if you know how much space there is in a van and what the maximum safe load the van can carry is, you can work out whether filling it with a specific material will be safe. You could also use the original version of the equation to work out what the densest material you could safely transport is.
Related Articles
Also Check: How Many Physics Questions Are On The Mcat
How Does Concentration Influence Optical Density
Since optical density can influence the speed of light due to optical absorption, it is quite evident that the concentration can also influence the optical density of the matter. If the optical density of the material is higher in value then it will decrease the speed of light and this causes the light to change its motion. Due to the slower speed of light, it will bend. Optical density is influenced by the concentration of light due to optical absorption. With increased concentration, the optical density of the matter will also increase.
Density Of Nuclear Matter
Nuclear density is the density of the nucleus of an atom. It is the ratio of mass per unit volume inside the nucleus. Since atomic nucleus carries most of atoms mass and atomic nucleus is very small in comparison to entire atom, the nuclear density is very high.
The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and from its mass. Typical nuclear radii are of the order 1014 m. Assuming spherical shape, nuclear radii can be calculated according to following formula:
r = r0 . A1/3
where r0 = 1.2 x 10-15 m = 1.2 fm
For example, natural uranium consists primarily of isotope 238U , therefore the atomic mass of uranium element is close to the atomic mass of 238U isotope . Its radius of this nucleus will be:
r = r0 . A1/3 = 7.44 fm.
Assuming it is spherical, its volume will be:
V = 4r3/3 = 1.73 x 10-42 m3.
The usual definition of nuclear density gives for its density:
nucleus = m / V = 238 x 1.66 x 10-27 / = 2.3 x 1017 kg/m3.
Thus, the density of nuclear material is more than 2.1014 times greater than that of water. It is an immense density. The descriptive term nuclear density is also applied to situations where similarly high densities occur, such as within neutron stars. Such immense densities are also found in neutron stars.
Also Check: Four Main Areas Of Biological Contamination
Give Each Student An Activity Sheet
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms & Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually, depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
Lead A Discussion About Why The Copper Cube Has A Greater Mass Than The Aluminum Cube
Tell students that both cubes are exactly the same size and both are solid with no hollow spots. Explain that the aluminum cube is made of only aluminum atoms and the copper cube is made of only copper atoms.
Ask students:
- How can two objects, which are exactly the same size and shape, have a different mass?
- Help students understand that the difference in mass must have something to do with the atoms in each cube. There are three possible explanations about the copper and aluminum atoms in the cubes that could explain the difference in mass.
Explain that any one of these explanations alone, or two or three together, could be the reason why the copper cube has more mass.
Also Check: Is Michael Jackson The Biological Father Of His Kids
Corrosionpedia Explains Mass Density
To simplify the comparison of densities of different materials across different units of measurement, other terms such as relative density or specific gravity are used. These dimensionless parameters represent the ratio of the mass density of a material to that of a standard material, typically water. Therefore, if the relative density of an object is less than one, that means that it is less dense than water and will likely float.
As mentioned previously, density varies depending on environmental temperature and pressure. As such, densities for various materials and elements are usually measured and recorded at standard conditions for temperature and pressure , i.e., 273.15°K and 100 kPa .
Increasing the pressure on an object or substance almost always increases its density. For example, a sponge ball starts with a particular density. If you were to squeeze the ball in your hand, its weight remains the same however, its volume changes as it is being compressed. As the same mass is contained in a smaller volume, based on = M/V, its density will increase.
The inverse is true for the density of materials subjected to changing temperature. Increasing the temperature of a substance almost always decreases its density due to expansion and increasing volumes. There are, however, a few notable exceptions. For example, water increases in density between its melting point at 0°C and 4°C.
Densities of Some Common Materials
| Material |
Show Animations And Demonstrate How To Measure Volume And Mass Of A Cube
Explain to students that volume is a measure of the amount of space an object takes up. It is always in three dimensions. To find the volume of an object like a cube or a box, you measure the length, width, and height and then multiply them . If measured in centimeters, the answer will be in cubic centimeters .
Note: Students often confuse volume and area. Check their understanding to make sure they know the difference. Make sure they understand that area is measured in two dimensions with an answer in cm2. Area is a measure of the amount of surface. But volume is measured in three dimensions with an answer in cm3. Volume is a measure of the entire object, including the surface and all the space the object takes up.
While the animation is playing, you can demonstrate the measuring process with a cube and ruler. Have students measure along with you to confirm the volume of the cubes.
Explore
Don’t Miss: New England Colony Climate
Density Atomic Mass And Atomic Number Density
Since the density of a substance is the total mass of that substance divided by the total volume occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density ,
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Therefore it is determined by the mass number .
- Atomic Number Density. The atomic number density , which is associated with atomic radii, is the number of atoms of a given type per unit volume of the material. The atomic number density of a pure material having an atomic or molecular weight and the material density is easily computed from the following equation using Avogadros number :
Density And Other Intensive Properties
The density of a material is strongly connected to other intensive properties, particularly temperature . Many materials expand when they are heated. Because a material that expands takes up a larger volume, its density decreases. This phenomenon occurs in all forms of matter: for example, solids, liquids, and gases. The tightly coupled relationship between density and temperature explains how hot air balloons work. When the air inside of a balloon is heated it expands and its density decreases. The balloon thus gains positive buoyancy with respect to the colder air surrounding it, and it floats into the sky.
Density is a fundamental physical property of matter. It is commonly used as a means of categorizing and identifying different materials. In addition, a thorough understanding of the concept of density is critical for building ships and lighter-than-air craft such as hot air balloons.
Read Also: How Old Are Elton Johns Kids
Notes About The Materials
Cubes
For this lesson, you will need a set of cubes of different materials that are all the same volume. These sets of cubes are available from a variety of suppliers. Flinn Scientific sells a Density Cube Set, Product #AP6058. This set comes with 10 cubes4 metal, 3 plastic, and 3 wood. It is easier for students if you reduce the number to 8 by using all the samples of metal but only two wood and two plastic cubes. We suggest using the nylon plastic cube and the PVC plastic cube. For the wood, we suggest using the oak and either the pine or poplar . In the activity, each group will need to measure the mass of each of the eight cubes. Groups will need to measure and record their data for a cube and pass it along to another group until each group has used each of the cubes.
Balances
Use a simple, plastic, two-sided balance that looks like a see-saw for the demonstration. One of the least expensive is Delta Education Primary Balance Product #WW020-0452. Have students use any balance that can measure in grams.
Metric ruler
Students will use a metric ruler in the engage portion of the activity when they measure the length, width, and height of a cube along with you.