What Are The Electron And Molecular Geometry Of H2s
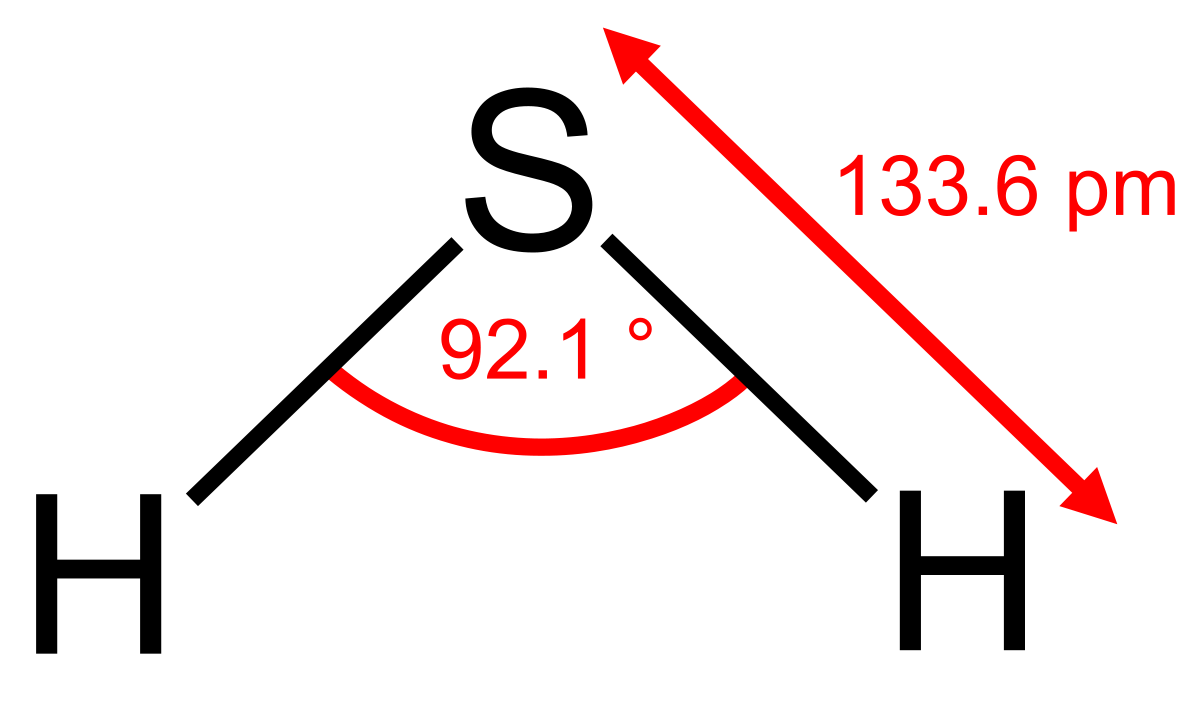
The molecular geometry of H2S is bent. This is because the two lone pair electrons on sulfur central atom repel each other as well as adjacent bonded pair electrons, as a result, these electron pair takes the position where the repulsion becomes minimum and they attains the stability.
Hence, the final shape or molecular geometry of H2S appears like a bent structure or V-shaped.
According to the VSEPR theory, to reduce the repulsion. the electron pairs move to maximum angle apart from each other and become stable.
Also, electron geometry for H2S is tetrahedral because 4 electrons which make 2 lone pairs around a sulfur atom are arranged in a tetrahedral geometry.
H2so4 Molecular Shape And Polarity
The molecular shape of a molecule and its polarity are dependent on the atoms involved in its formation. The electronic configuration including lone pairs gives the most stable shape to the molecule.
The electronegativity of atoms and the type of bond formation determine whether a molecule has polar or nonpolar nature.
Molecular Shape
in the H2SO4 molecule, Sulfur being the central atom has four paired electrons and 0 unpaired electrons.
Hence, its structure is classified as a tetrahedron with 109.5 degrees between the Sulfur and the four Oxygen atoms.
Hydrogen and Oxygen form a linear bond with an angle of 180 degrees.
Molecular Polarity
The H2SO4 molecule is polar in nature because of the bent H-O-S bonds which are present in it. As a result, the charge distribution across the molecule becomes non-uniform.
Molecular Geometry Of If2^
Expert Solution
Concepts and reason
The problem is based on the concepts of VSEPR theory and Lewis structure. This is used to tell the geometry molecules taking electron pairs surrounding their central atoms into consideration. Both bond pairs and lone pairs are contributing to determining the geometry of molecules.
Fundamentals
There are certain rules for writing Lewis structure of a molecule. These rules are as follows:
1. First, draw the molecule’s skeleton, keeping more electronegative atoms at the terminal position and less electronegative at the center.
2. Count the total number of valance electrons by taking the sum of each atom’s valence electron.
3. Distribute electrons in such a way that the octet of every atom is complete.
4. Electrons that are not taking part in bonding are represented as dots and are known as lone pairs of that atom.
In \, both iodine and fluorine belongs to the halogen family and have 7 electrons in the outermost valence shell. Since fluorine is more electronegative than iodine, iodine will be at the center. There are 7 valence electrons in iodine also 1 negative charge adds one more electron thus, the total number of electrons surrounding iodine will be 8 as follows:
Two fluorine atoms can share 2 of the 8 electrons resulting \ hybridization as follows:
According to VSEPR theory, \ hybridization corresponds to trigonal bipyramidal geometry , which is represented as follows:
Molecular geometry of \ is Linear
Don’t Miss: Who Is Paris Jackson’s Real Father
Calculating Lone Pair Of Electrons On Hydrogen In The H2s Geometry:
Finding lone pair of electrons for the terminal hydrogen atom is not similar to the central sulfur atom. We use the following formula as given below
Use the formula below to find the lone pair on the hydrogen atom of the H2S molecule.
L.P = V.E â N.A
Lone pair on the terminal hydrogen atom in H2S = L.P
Terminal hydrogen atomâs valence electron in H2S = V.E
Number of S-H bonds = N.A
calculation for hydrogen atom lone pair in H2S molecule.
For instance of H2S, their terminal atoms, hydrogen, have one electron in its outermost valence shell, one S-H single bond connection. This gives a total of two S-H single bond connections. But we are considering only one connection for the calculation.
As a result of this, L.P = =0
The lone pair of electrons in the hydrogen atom of the H2S molecule is zero. Two hydrogen atoms are connected with the central sulfur atom.
In the H2S electron geometry structure, the lone pairs on the central sulfur atom are two, lone pairs of electrons in the hydrogen atom have zero. Two hydrogen atoms have no lone pairs of electrons.
It means there are two lone pairs of electrons in the core sulfur atom. Two lone pair of electrons on the central sulfur atom is responsible for the tetrahedral nature of H2S molecular geometry. But in the structure hydrogen atoms are polarised sidewise in their tetrahedral geometry.
What Is The Molecular Geometry Of Brf5
Hydrogen sulphide has a linear molecular geometry, meaning that its molecular bonds run along one straight line. This molecule also has two unpaired electrons. Chlorine trifluoride has a linear molecular geometry with three unpaired electrons in its outer shll.
Brmine pentafluoride has a linear structure with five unpaired electrons on its outer surface. Chloroethane and hydrocyanic acid have trigonal pyramidal structures these molecules have three bonds running between each pair of atoms, creating an overall pyramid shape. Two different atoms are bonded at each of two opposite corners.
Don’t Miss: Cpm Algebra 2 Chapter 1 Answers
Is H2s Polar Or Non
Well, we know the polar molecule has some dipole moment because of unequal distribution of charges whereas the non-polar molecule has an equal distribution of charges that cause zero dipole moment because they cancel out each other due to the symmetrical shape of the molecule.
The polarity of H2S is dependent upon its molecular shape, electronegativity, and dipole moment.
H2S is non-polar because the electronegativity of bonds is lesser than 0.5, as Hydrogen electronegativity is 2.20 and sulfur electronegativity is 2.58 and their difference is 0.38 which causes H2S to become nonpolar in nature.
According to the Pauling scale, if the difference of an atoms electronegativity is less than 0.4, the bond is considered nonpolar and vice-versa.
H2S can also assume to be a polar molecule because it is non-linear and it has bent molecular geometry and central atom S is more electronegative than H, therefore, the negative pole is on the S atom. So, H2S has some dipole moment which makes it a polar molecule.
Technically, due to the very weak polarity nature of H2S, it is considered a non-polar molecule.
The dipole moment of H2S is 0.95D and in the mathematical term, it can be expressed as-
product of charges of two atoms and the distance between them
D = Q * R
Q denotes charge on atoms
R denotes the distance between atoms
Uses of Hydrogen Sulfide
Properties of Hydrogen Sulfide
FeS + 2 HCl FeCl2 + H2S
6 H2O + Al2S3 3 H2S + 2 Al3
Type Of Bonding In H2so4
This is a bisulfate anion that takes electrons from other metals. Hydrogen, sulfur, and oxygen atoms form covalent polar bonds.
This further means that the dipole moment is in effect, leading to which compound has a positive and negative charge to it.
In addition to this sulfur has high electronegativity and so does oxygen. This is the reason why electrons are closer to these two elements instead of hydrogen.
As we can see in the Lewis Structure, H2SO4 takes sulfur as the center atom.
Two of the oxygen atoms are double-bonded to the sulfur atom, while the rest of the two are single-bonded.
Likewise, a hydrogen atom is attached to each single-bonded oxygen atom.
Also Check: Steve Harvey Oldest Son
What Is The Molecular Geometry Of H2s Clf3 Brf5 Clo2 Ch4 H2co Icl2 And Bro3
We will look only at the molecular systems of some common molecules throughout this blog post. The shape of a molecule can tell you a lot about how a molecule will behave. Some molecules are linear, some are cyclic, and some are bent.
The following molecules are all linear. Water, HCl, and SiF4 are all linear. I2 and BrF3 are both linear, but BrF3 is bent. The following molecules are cyclic. Chlorine, Chlorine Dioxide, and Bromine are all cyclic. They are not linear because the central atom is surrounded by two other atoms and two other bonds. The following molecule is bent. Hydrogen Chloride is bent because two different particles and three bonds surround the central atom.
We briefly discussed how molecular geometry determines how molecular bonds form and react with other substances in an earlier article. This article will get more specific by looking at the geometry of six common gases, specifically h2s, clf3, brf5, clo2, ch4 and h2co . Well also discuss bro3 since it has the same chemical formula as h2co but has different properties due to its crystalline structure.
How To Draw H2s Lewis Structure
The hydrogen sulfide chemical formula is H2S. Drawing H2S Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct H2S Lewis Structure. The sulfur and hydrogen elements come as the member of the oxygen and hydrogen family groups from the periodic table respectively. The valence electrons in sulfur and hydrogen are six and one respectively. Hydrogen sulfide is used to make chemical reagents for organic chemical reactions for the production of sulfur-organic materials.
Recommended Reading: Geometry Segment Addition Postulate Worksheet Answer Key
Draw The Lewis Dot Structure Of Hydrogen Sulphide Molecule
10 Lakh+ Solutions, PDFs, Exam tricks!
Play here
Get Answer to any question, just click a photo and upload the photoand get the answer completely free,UPLOAD PHOTO AND GET THE ANSWER NOW!
Text Solution
Answer
Apne doubts clear karein ab Whatsapp par bhi. Try it now.
Ab clear karein apne doubts Whatsapp par bhi. Apna phone number register karein.
Ab aap Whatsapp pe solutions paa saktey h, hum aapko message karenge.
Hurray!! Ab aap Whatsapp pe solutions paa saktey h, hum aapko ping karenge
Lewis Dot Structure Of H2so4
Follow the step-by-step procedure to draw the H2SO4 Lewis Dot Structure.
Step 1 H2SO4 Valence Electrons
To draw the Lewis structure of H2SO4 we will first have to determine the number of valence electrons in the molecule.
Sulfur being a group 16 element has 6 valence electrons and requires 2 electrons to complete its octet, while Oxygen which is also a group 16 element contains 6 electrons in its outermost shell and therefore, needs two electrons.
The hydrogen atom has 1 electron in its valence shell and requires one more electron to reach the stable state.
Therefore, the total number of electrons for H2SO4 is:
Sulphur = 6 Valence electrons
Oxygen = 6 Valence electrons for 4 Oxygen atoms, 4 X 6 = 24
Hydrogen = 1 Valence electron for 2 Hydrogen atom, 2 X 1 = 2
Therefore, total number of valence electrons in H2SO4 = 32
Step 2 The electrons from the hydrogen atom may also be considered as 2 negative charges as they do not have much role to play in the structure.
Step 3 Drawing the basic structure Sulphur is taken as the central atom which is joined with all the four oxygen atoms through the single bond to calculate the number of electrons further required to complete the octet of the given atoms.
Step 4 Now looking at the structure it is clear that two oxygen atoms, as well as the sulfur atom, have a complete octet.
Although it is seen here that sulfur after the formation of four single bonds now has ten electrons in its valence shell.
Formal Charge =
Recommended Reading: Paris Jackson’s Father
Molecular Models Diagram Of Water
The molecular model diagram is a diagram that depicts the process of deciding chemical bonding between molecules in a compound.
The molecular model diagram also aids in determining how two sigma bonds are formed as well as the influence of lone pairs on the structure.
The six valence electrons bond with the hydrogen atoms 1s orbital electrons, as can be seen in the diagram above.
The combining and overlapping of atomic orbitals of varying energies takes place.
Its happening in such a way that lower-energy bonding electrons are forming higher-energy antibonding molecular orbitals.
Owing to a lack of electrons, the left oxygen electrons do not overlap any more.
The electronegativity of the oxygen atom is greater than that of hydrogen.
As a result, oxygen has a higher negative charge than hydrogen, which is positive. It causes oxygen to draw neighbouring electrons and, eventually, form a bond.
The hydrogen, on the other hand, does not react with surrounding molecules because it has already completed its orbital and is bound to oxygen by a sigma bond that is difficult to sever.
It causes polarity to form in an H2O molecule, regardless of whether it has a net neutral charge.
You may also be interested in reading an essay on water polarity.
What Are The Valence Electrons Of Hydrogen And Oxygen
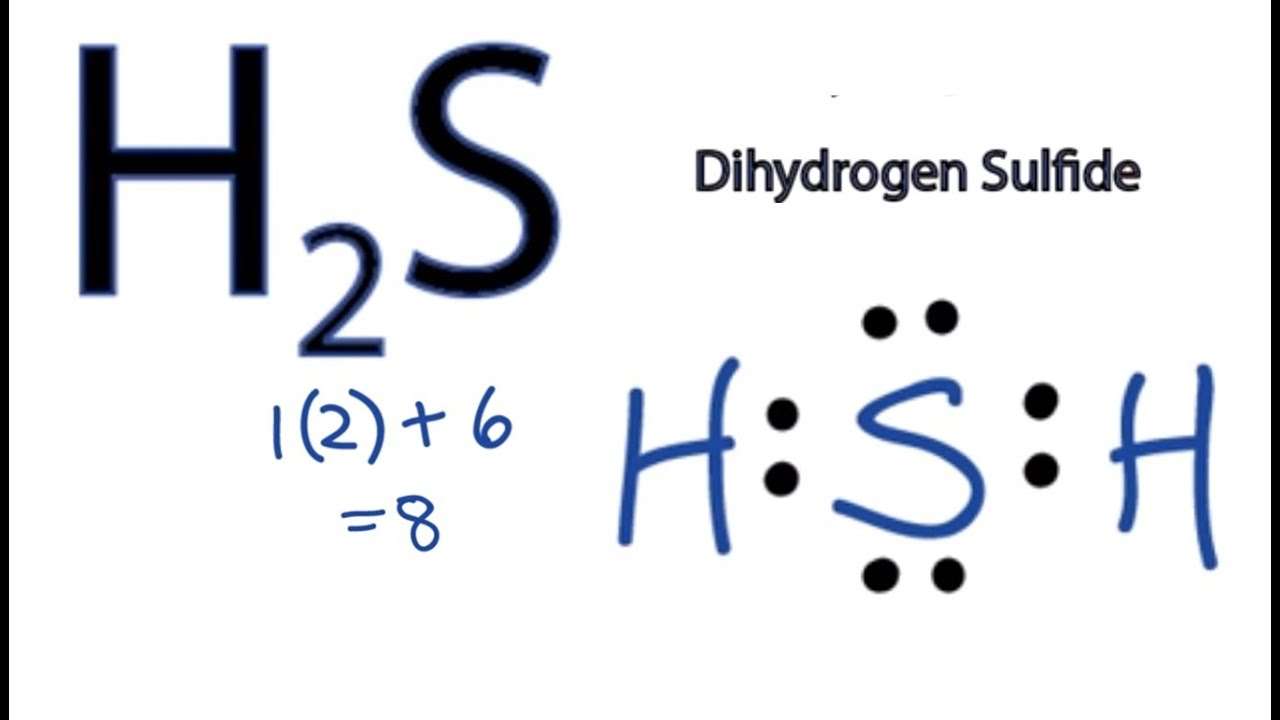
The valence electrons are the free electrons in the atoms outermost casing. Since it is the farthest out, the nucleus retains the outer shell with a shaky grip.
In addition, unpaired valence electrons become extremely reactive in nature, taking or contributing electrons to maintain the outermost shell.
Its worth noting that the greater the number of valence electrons, the more powerful the ability to accept electrons.The atoms capacity to donate valence electrons increases as the number of valence electrons decreases.
Read Also: Holt Mcdougal Geometry Worksheet Answer Key
H2s Molecular Geometry And Shape
A molecules molecular geometry refers to the three-dimensional arrangement of the atoms that make up the molecule. The study of any molecules molecular geometry is important because it provides information on many physical and chemical properties of the compound such as polarity, reactivity, phase of matter, color, magnetism, biological activities, and so on.
H2S molecule has a bent shape. Hydrogen sulfide molecule has two H S bonds and two lone pairs of electrons on central atom sulfur. These two bonds and two lone pairs of electrons make up four regions of electron density around the central sulfur atom. These four regions of electron density make the shape of H2S bent or V-Shaped.
We can also determine the geometry and shape of the H2S molecules using the VSEPR theory. For this, we can use the AXN method.
AXN Notation for H2S molecule:
- A represents the central atom, In the H2S molecule, the central atom is sulfur. So, A = Sulfur
- X represents the bonded atoms to the central atom. In H2S molecule, 2 hydrogen atoms are bonded to the central atom Sulfur. So, X = 2
- N represents lone pair of electrons on the central atom. The central atom Sulfur has 2 lone pairs of electrons.
So the AXN generic formula for H2S becomes AX2N2.
According to VSEPR theory, if the molecule has an AX2N2 formula then the molecule has bent molecular geometry with tetrahedral electron geometry.
Molecular Geometry Notation For H2s Molecule :
Determine the form of H2S molecular geometry using VSEPR theory. The AXN technique is commonly used when the VSEPR theory is used to calculate the shape of the H2S molecule.
The AXN notation of H2S molecule is as follows:
The central sulfur atom in the H2S molecule is denoted by the letter A.
The bound pairs of electrons to the core sulfur atom are represented by X.
The lone pairs of electrons on the central sulfur atom are denoted by the letter N.
Notation for H2S molecular geometry
We know that H2S is the core atom, with two electron pairs bound and two lone pairs of electrons. The general molecular geometry formula for H2S is AX2N2.
According to the VSEPR theory, if the H2S molecule ion has an AX2N2 generic formula, the molecular geometry and electron geometry will both be tetrahedral or V-bent-shaped forms.
| Name of Molecule | |
| The formal charge of H2Son sulfur | 0 |
You May Like: Holt Algebra 2 Chapter 7 Test Answer Key
Total Number Of Electrons Of The Valance Shells Of H2o
There are two of elements hydrogen and oxygen. Hydrogen is a group IA element and has only one electron in its last shell . Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. Now we know how many electrons are includes in valence shells of each atom.
- valence electrons given by hydrogen atoms = 1 * 2 = 2
- valence electrons given by oxygen atoms = 6*1 = 6
- Total valence electrons = 2 + 6 = 8
What Is The Molecular Geometry Of Ch4
Because, according to VSEPR theory, molecular geometry considers only bond pairs or atoms, while electron geometry considers bonded atoms and lone pairs present on the central atom, CH4s molecular geometry and electron geometry are tetrahedral.
The molecule H2 is a diatomic molecule with the chemical formula H2. It exists at low concentrations as a gas in Earths atmosphere. It is the simplest example of a homonuclear diatomic molecule that is non-linear. This means that the two atoms forming the molecule are connected by a bond that is not a simple straight line.
The molecular geometry of methane is of interest to researchers in many fields. Indeed, it is helpful in organic chemistry and biochemistry as a model of the effects of various substituents on the geometry of the carbon atom. For example, the results of a methyl group on the geometry of the carbon atom have been extensively studied.
You May Like: Holt Geometry Chapter 7 Test Form B Answers
What Is The Molecular Geometry Of Clf3
The bond angle in any molecule can be described using VSEPR theory. In the VSEPR view, we use electron bonds to describe how valence electrons are distributed around an atom in a molecule.
So when we look at ClF3, its a highly electronegative fluorine that determines where its electrons will be on Cl-F-Cl bonds. It has three pairs of unshared valence electrons outside its outermost shell. The goal of bonding is to get as many shared pairs as possible. With that in mind, fluorine would want to approach two other fluorines as closely as possible for maximum bonding capability.