Algorithm Of Assigning Bonds
This algorithm is performed on a Lewis structure . Oxidation state equals the charge of an atom after each of its heteronuclear bonds has been assigned to the more-electronegative partner of the bond and homonuclear bonds have been divided equally:
where each “” represents an electron pair , and “OS” is the oxidation state as a numerical variable.
After the electrons have been assigned according to the vertical red lines on the formula, the total number of valence electrons that now “belong” to each atom is subtracted by the number N of valence electrons of the neutral atom to yield that atom’s oxidation state.
This example shows the importance of describing the bonding. Its summary formula, HNO3, corresponds to two structural isomers the peroxynitrous acid in the above figure and the more stable nitric acid. With the formula HNO3, the simple approach without bonding considerations yields 2 for all three oxygens and +5 for nitrogen, which is correct for nitric acid. For the peroxynitrous acid, however, the two oxygens in the OO bond each has OS = 1 and the nitrogen has OS = +3, which requires a structure to understand.
Organic compounds are treated in a similar manner exemplified here on functional groups occurring in between CH4 and CO2:
Analogously for transition-metal compounds CrO2 on the left has a total of 36 valence electrons , and Cr6 on the right has 66 valence electrons :
The algorithm’s caveat
How Do I Find The Oxidation Number Of A Coordination Complex
I actually know how to calculate the oxidation number for coordination complexes but some complexes like the below are giving some reistance
$\ce$ triaquoferrate)
I just wrote this example now and don’t know wether it exists or not I am rather concerned about how do I find their oxidation numbers
Think of it like this. Ammonia and Water are both neutral molecules, therefore they won’t contribute to the charge of the two coordination spheres. There are also no subscripts, which indicates that iron and copper both have the same magnitude of charge. Now copper here could be +1 and iron could be in -1, or copper in +2 and iron in -2. I cannot conclusively say which though, without any further data, such as maybe their spin only angular momenta.
For other complex compounds, i suggest you practice, and eventually you’ll get the hang of what oxidation states are common for different metals. Also use the concepts you use for normal covalent compounds. Trust me, it’s very easy!
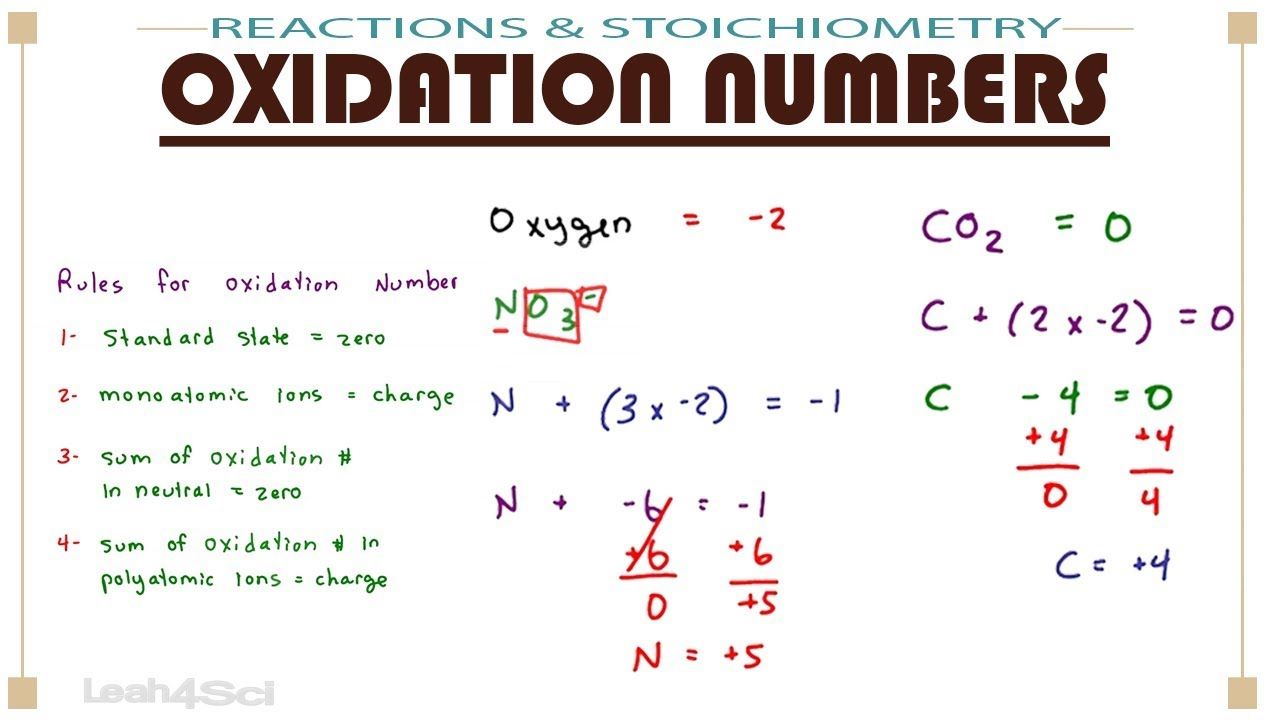
Assigning Oxidation Numbers Based On Chemical Rules
You May Like: Punchline Bridge To Algebra Answer Key Page 115
Same Old Same Old: Some Elements Form Typical Fixed Oxidation State
When elements react to form compounds, their oxidation state changes.
| Element | |
|---|---|
| +1 | |
| Group I metals | |
| Fluorine | -1 |
Some elements mainly form one type of oxidation state in compounds. We say that they have fixed oxidation state. For example, hydrogen has a fixed oxidation state of +1 in all covalent compounds.
Likewise, fluorine is pretty boring. In all fluorine-containing compounds, fluorine has a fixed oxidation state of -1.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Read Also: What Are 4 Goals Of Psychology
Oxidation Number Of Atoms In A Diatomic Molecule
A diatomic molecule can be either homo or heteronuclear.
i) Homonuclear diatomic molecule:
Oxidation number concept is applicable only to heteroatoms forming a molecule. Hence, in a homonuclear diatomic molecule, the oxidation number of the atoms is zero. The oxidation number of hydrogen or oxygen, nitrogen, chlorine in respective molecules is zero.
ii) Heteronuclear diatomic molecule:
In hetero diatomic molecules, all bonds formed between the atoms are, considered as ionic.
More electronegative atoms are assumed to take away the bonding electrons from the less electronegative atom. So, the electronegative atom will have a negative oxidation state and the magnitude is equal to the number of electrons taken by it.
The less electronegative atom is supposed to have lost its electron to the more electronegative atom. So, the less electronegative atom will have a positive oxidation state equal to the number of electrons lost by it.
Example 1: HCl
Chlorine is highly electronegative than hydrogen. So, chlorine is, assumed to take away the electron from hydrogen. Chlorine, which receives one electron, has an oxidation number of -1, while hydrogen losing one electron has an oxidation state of +1.
Example 2: H2O
Oxygen is more electronegative than hydrogen. So, the oxygen atom receives one electron each from the two-hydrogen atom and will have an oxidation number of -2. Both hydrogens losing one electron each will have an oxidation number of +1 each.
Oxidation Number Definition Rules Calculation Examples
Redox is a chemical process that involves changing the oxidation states of atoms. The actual or formal transfer of electrons between chemical species is defined by redox reactions, which usually include one species suffering oxidation while another species experiences reduction . The chemical species that loses an electron is said to have been oxidised, whereas the chemical species that gains an electron is said to have been reduced. To put it another way:
The loss of electrons or a rise in the oxidation state of an atom, an ion, or specific atoms in a molecule is referred to as oxidation.
The gain of electrons or a drop in the oxidation state of an atom, an ion, or specific atoms in a molecule is referred to as reduction .
You May Like: What Causes Parallax Error
Oxidation And Reduction Reaction Example Problem
In an oxidation-reduction or redox reaction, it is often confusing to identify which molecule is oxidized in the reaction and which molecule is reduced. This example problem shows how to correctly identify which atoms undergo oxidation or reduction and their corresponding redox agents.
Periodic Table With Oxidation Number
Some general rules are used for the calculation of the oxidation numbers of s, p, d, and f-block elements in the periodic table. The s-block elements commonly show +1 and +2 oxidation numbers but p-block elements commonly show +3, +4, -3, -2 and -1 oxidation numbers. One of the most important properties that distinguish transition metals or d-block elements from non-transition elements is variable oxidation numbers or states.
Read Also: Kuta Software Infinite Algebra 2 Operations With Complex Numbers
Algorithm Of Summing Bond Orders
This algorithm works on Lewis structures and bond graphs of extended solids:
Oxidation state is obtained by summing the heteronuclear-bond orders at the atom as positive if that atom is the electropositive partner in a particular bond and as negative if not, and the atoms formal charge is added to that sum.
Applied to a Lewis structure
An example of a Lewis structure with no formal charge,
illustrates that, in this algorithm, homonuclear bonds are simply ignored .
Carbon monoxide exemplifies a Lewis structure with formal charges:
To obtain the oxidation states, the formal charges are summed with the bond-order value taken positively at the carbon and negatively at the oxygen.
Applied to molecular ions, this algorithm considers the actual location of the formal charge, as drawn in the Lewis structure. As an example, summing bond orders in the ammonium cation yields 4 at the nitrogen of formal charge +1, with the two numbers adding to the oxidation state of 3:
The sum of oxidation states in the ion equals its charge .
Also in anions, the formal charges have to be considered when nonzero. For sulfate this is exemplified with the skeletal or Lewis structures , compared with the bond-order formula of all oxygens equivalent and fulfilling the octet and 8 N rules :
Applied to bond graph
How To Find Oxidation Number Of An Atom
Oxidation number or state of an atom/ion is the number of electrons an atom/ion that the molecule has either gained or lost compared to the neutral atom. Electropositive metal atoms, of group I, 2 and 3 lose a specific number of electrons and have always constant positive oxidation numbers.
In molecules, more electronegative atom gain electrons from a less electronegative atom and have negative oxidation states. The numerical value of the oxidation state is equal to the number of electrons lost or gained.
Oxidation number or oxidation state of an atom or ion in a molecule/ion is assigned by:
i) Summing up the constant oxidation state of other atoms/molecules/ions that are bonded to it and
ii) Equating, the total oxidation state of a molecule or ion to the total charge of the molecule or ion.
Recommended Reading: Who Is The Mother Of Paris Jackson
Oxidation State In Metals
Many compounds with luster and electrical conductivity maintain a simple stoichiometric formula such as the golden TiO, blue-black RuO2 or coppery ReO3, all of obvious oxidation state. Ultimately, however, the assignment of the free metallic electrons to one of the bonded atoms has its limits and leads to unusual oxidation states. Simple examples are the LiPb and Cu3Au ordered alloys, the composition and structure of which are largely determined by atomic size and packing factors. Should oxidation state be needed for redox balancing, it is best set to 0 for all atoms of such an alloy.
Simple Approach Without Bonding Considerations
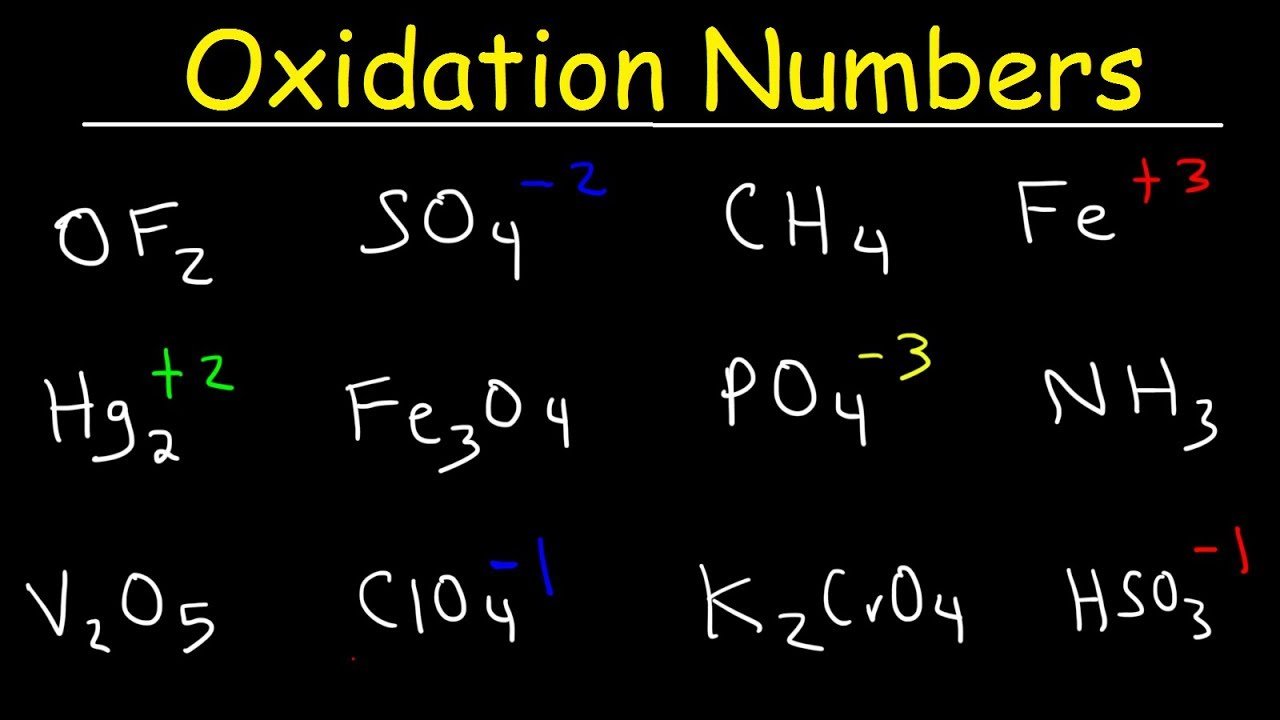
Introductory chemistry uses postulates: the oxidation state for an element in a chemical formula is calculated from the overall charge and postulated oxidation states for all the other atoms.
A simple example is based on two postulates,
where OS stands for oxidation state. This approach yields correct oxidation states in oxides and hydroxides of any single element, and in acids such as H2SO4 or H2Cr2O7. Its coverage can be extended either by a list of exceptions or by assigning priority to the postulates. The latter works for H2O2 where the priority of rule 1 leaves both oxygens with oxidation state 1.
Additional postulates and their ranking may expand the range of compounds to fit a textbook’s scope. As an example, one postulatory algorithm from many possible in a sequence of decreasing priority:
You May Like: Kuta Software – Infinite Algebra 2 Rationalizing Imaginary Denominators
Calculating Oxidation States In Inorganic Compounds
Now heres a fun exercise. Try applying the same rules to carbon.
Its going to feel a little bit weird. Why? Because there are two key differences.
- First, carbon is often more electronegative than some of the atoms its bound to . So what do you do in this case?
- Secondly, unlike metal-metal bonds, carbon-carbon bonds are ubiquitous. So how do you deal with them?
Two answers.
So unlike metals, which are almost always in a positive oxidation state, the oxidation state of carbon can vary widely, from -4 to +4 . Here are some examples.
How To Use An Oxidation Number Calculator
To use the oxidation number calculator, you only have to follow a few basic steps as follows
- Write the chemical formula of the compound accurately. While writing the chemical formula, pay special attention to capital and small letters. If you write CAOH2 rather than CaOH, the calculator will not detect the substance.
- Write the correct number of atoms of each element present in the compound. Remember, if you skip or mistakenly add more atoms of an element, the entire nature of your substance will change. Eventually, the oxidation number of each reacting element will be altered. To confirm the correct number of atoms in the compound, you can use our equation balancer.
- Avoid using unnecessary brackets or parenthesis while writing chemical formulas.
- After inserting the formula, click on the balance or calculate button and wait a few seconds.
Don’t Miss: Prince And Paris Jackson Biological Father
Calculation Of Oxidation Number Of Atoms Occurring More Than Once In A Molecule And Having A Difference In Bonding
Atoms having different bond structure will have different oxidation state. Hence, their oxidation state has to be individually determined from their molecular structure. Average oxidation state can be calculated by assuming them to be equal. In such a case, the average oxidation could be fractional rather than a whole integer.
Example 1: Cl2O4
i) The average oxidation state of chlorine
Cl2O4 is neutral and so, net charge = 0
Oxidation state of Cl2O4 = 2 x Oxidation state of chlorine + 4 x oxidation state of oxygen = 0.
Oxidation state of oxygen = -2.
Oxidation states 2x + = 0: x = +4
Atoms in the species 2Cl 4O
Oxidation state of chlorine in Cl2O5 = 8
56 = fraction
ii) Without resonance, four carbon has -1 oxidation state and one carbon has -2 oxidation state.
How To Find Oxidation Numbers
wikiHow is a wiki, similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 37 people, some anonymous, worked to edit and improve it over time.There are 7 references cited in this article, which can be found at the bottom of the page.wikiHow marks an article as reader-approved once it receives enough positive feedback. This article has 48 testimonials from our readers, earning it our reader-approved status. This article has been viewed 1,092,136 times.Learn more…
In chemistry, the terms “oxidation” and “reduction” refer to reactions in which an atom loses or gains electrons, respectively. Oxidation numbers are numbers assigned to atoms that help chemists keep track of how many electrons are available for transfer and whether given reactants are oxidized or reduced in a reaction. The process of assigning oxidation numbers to atoms can range from remarkably simple to somewhat complex, based on the charge of the atoms and the chemical composition of the molecules they are a part of. To complicate matters, some elements can have more than one oxidation number. Luckily, the assignment of oxidation numbers is governed by well-defined, easy-to follow rules, though knowledge of basic chemistry and algebra will make navigation of these rules much easier.XResearch source
Don’t Miss: Unit 1 Test Study Guide Geometry Basics Gina Wilson
Elements With The Most Oxidation States
Vanadium, manganese, and chromium have the greatest variety of stable oxidation states and colors. The cover photo for this article, taken by Wilco Oelen , shows the colors of vanadium compounds in the +2, +3, +4 and +5 oxidation states. Tungsten and molybdenum also have several oxidation states, some of which are less studied than other transition metals.
Fun fact the highest known oxidation state is reported to be +9 in the tetroxoiridium cation .