Read A Brief Summary Of This Topic
hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon and hydrogen . The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. Hydrocarbons are the principal constituents of petroleum and natural gas. They serve as fuels and lubricants as well as raw materials for the production of plastics, fibres, rubbers, solvents, explosives, and industrial chemicals.
Many hydrocarbons occur in nature. In addition to making up fossil fuels, they are present in trees and plants, as, for example, in the form of pigments called carotenes that occur in carrots and green leaves. More than 98 percent of natural crude rubber is a hydrocarbon polymer, a chainlike molecule consisting of many units linked together. The structures and chemistry of individual hydrocarbons depend in large part on the types of chemical bonds that link together the atoms of their constituent molecules.
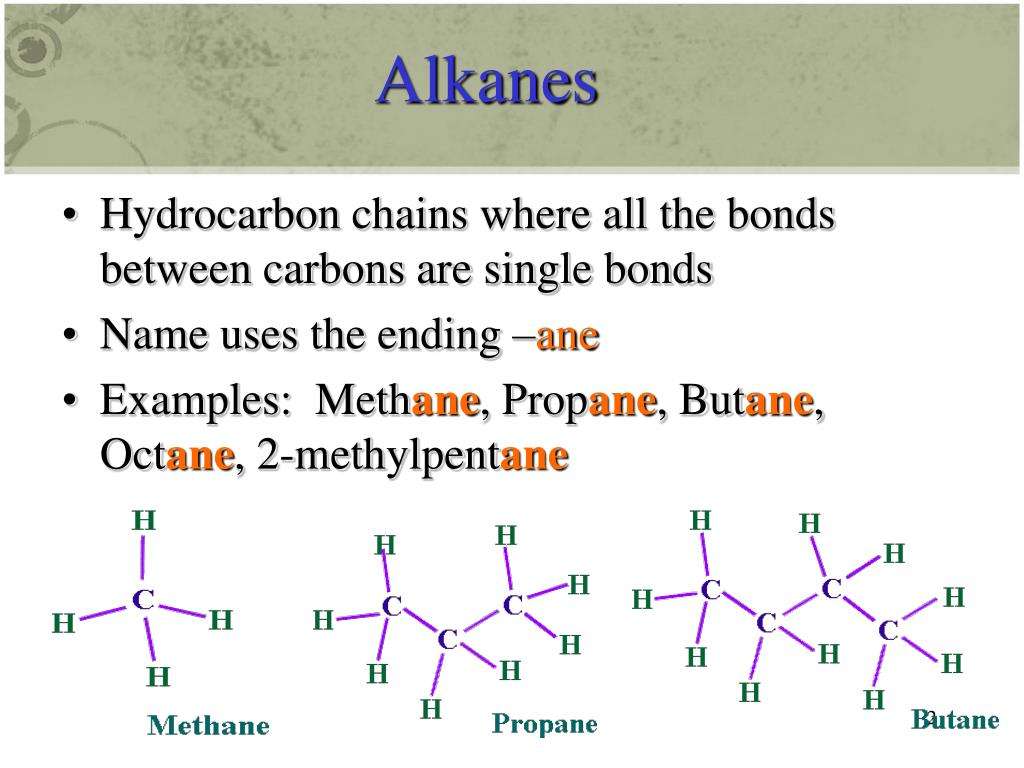
Alkanes are described as saturated hydrocarbons, while alkenes, alkynes, and aromatic hydrocarbons are said to be unsaturated.
Iupac Nomenclature For Branched
The following rules should be kept in mind when using the IUPAC technique to name saturated hydrocarbons with branching chains.
What Are Homologous Series
In the same way that all elements with similar electron structures have similar chemical properties and are grouped together in the same periodic table group, all organic compounds with similar structures have similar properties and are grouped together in the same group or series. The organic compounds are then organized in increasing molecular mass order.
A homologous series is a collection of organic compounds with identical structures and chemical characteristics that differ only in the CH2 group between them. Homologous refers to the different chemical molecules that make up a homologous series. The two neighbouring molecules are clearly separated by one carbon atom and two hydrogen atoms .
Homologous Series of alkanes- Since all alkanes have identical structures with single covalent bonds and chemical characteristics, they can be classified together in a homologous series. The first five alkanes in the homologous series are listed below.
| Alkanes | |
| Pentane | C5H12 |
The homologous series of alkanes have the general formula CnH2n+2, where n is the number of carbon atoms in one molecule of alkane. One carbon atom makes up the first member of the alkane series. The second alkane series member has two carbon atoms. The third alkane series member has three carbon atoms. The fourth alkane series member has four carbon atoms, while the fifth alkane series member has five carbon atoms.
| Alkenes |
| Hexyne | C6H10 |
Read Also: Abiotic Ecology Definition
Preparation Of Hydrocarbons Alkanes
From alkenes and alkynes
The alkanes can be produced from alkenes or alkynes through hydrogenation. H2 gas is passed over a metal surface such as Ni, Pt along with the alkenes to produce alkane.
CH2=CH2 CH3-CH3
The above reaction is called Sabatier-Sender sons reaction. Other catalysts which can be used are Pt, Pd-BaSo4, Adams catalyst or Wilkinson catalyst , etc.
From Alkyl Halides
Alkyl halides can be converted to alkanes through various methods. They are as follows.
1. Using Zn/Protic solvents
Note: Alkanes with only even number of carbons atoms can be produced.
3. Using Reducing Agents:
The reducing agents which can be used are LiAlH4, NaBH4, NaNH2, etc.
Note:
- LiAlH4 cant reduce 3° halides.
- NaBH4 cant reduce 1° halide.
2. From Aldehydes/Ketones:
Properties Of Polyaromatic Hydrocarbon Compounds
PAHs are lipophilic in nature, having higher affinity for organic matters and low affinity toward water are thermodynamically stable and possess a large negative resonance energy . As per the rule given by Clar , PAHs have a resonance structure with a large number of disjoint aromatic -sextet, i.e., benzene-like moieties, which is responsible for the very distinctive properties of PAHs . Different PAH compounds show diverse chemical and physical properties as shown in Table 19.1. Depending on the molecular weight, these compounds have been categorized into two groups, namely, low-molecular-weight PAHs and high-molecular-weight PAHs. The low-molecular-weight compounds are more volatile and water soluble than the high-molecular-weight PAHs, which are more soluble in organic solvents . The hydrophobicity of PAH compounds can be predicted with the help of octanol-water partition coefficient which is the ratio of a chemical’s concentration in the octanol phase to that in the aqueous phase of a two phase octanol/water system .
Table 19.1. Chemical Structures and Selected Properties of 16 USEPA Priority Pollutant Polyaromatic Hydrocarbons .
| Compound |
|---|
Richard J. Wenning, Linda Martello, in, 2014
Also Check: What Does Ppt Mean In Chemistry
Chemistry End Of Chapter Exercises
an alkane
2-pentyne
trans6-ethyl-7-methyl-2-octene
Draw Lewis structures for these compounds, with resonance structures as appropriate, and determine the hybridization of the carbon atoms in each.
1 mol of 1-butyne reacts with 2 mol of iodine.
Pentane is burned in air.
2-butene reacts with chlorine.
benzene burns in air.
How many kilograms of ethylene is produced by the pyrolysis of 1.000 × 103 kg of ethane, assuming a 100.0% yield?
Key Concepts And Summary
Strong, stable bonds between carbon atoms produce complex molecules containing chains, branches, and rings. The chemistry of these compounds is called organic chemistry. Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbonsthat is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Aromatic hydrocarbons contain ring structures with delocalized electron systems.
Recommended Reading: Houghton Mifflin Geometry Workbook Answers
Characteristic Of Homologous Series
The following are the properties of the Homologous Series.
- The chemical characteristics of all substances in a homologous series are similar. All alkane series molecules, such as methane, ethane, propane, and others, undergo substitution reactions with chlorine.
- A homologous series members can all be represented by the same general formula.
- In their molecular formulas, any two neighbouring homologous differ by one carbon atom and two hydrogen atoms.
- With increasing molecular mass, members of a homologous series display a steady change in their physical properties.
- Any two neighbouring homologous molecules have a molecular mass difference of 14u.
Classification And Types Of Hydrocarbons
Older chemists classified hydrocarbons as either aliphatic or aromatic. The classification was done based on their source and properties. As such, it was found that Aliphatic hydrocarbons were derived from the chemical degradation of fats or oils whereas aromatic hydrocarbons contained substances that were a result of the chemical degradation of certain plant extracts. However, today we classify hydrocarbons on the basis of structure and not merely on the origin.
Classification of Hydrocarbons
Don’t Miss: The Founder Of Behaviorism Was:
The Basics Of Organic Nomenclature: Naming Alkanes
The International Union of Pure and Applied Chemistry has devised a system of nomenclature that begins with the names of the alkanes and can be adjusted from there to account for more complicated structures. The nomenclature for alkanes is based on two rules:
Lesson Explainer: Hydrocarbons Chemistry
In this explainer, we will learn how to identify and name simple hydrocarbons and represent them using different types of formulas.
Organic chemistry is a branch of chemistry that studies carbon-based compounds, the simplest of which is the hydrocarbon. A hydrocarbon is a compound that is composed entirely of covalently bonded carbon and hydrogen atoms. Butane , a fuel used in lighters, octane , a component of gasoline, and naphthalene , a moth repellent, are all examples of hydrocarbons.
You May Like: Hrw Geometry Answers
Naming Of Unsaturated Hydrocarbons With Triple Bond
- The naming of C2H2 The letter eth denotes the presence of two carbon atoms in this molecule. This hydrocarbon is unsaturated because it has a carbon-carbon triple bond. The yne at the ending is used to denote a triple bond. When the letters eth and yne are combined, the IUPAC name for this combination is ethyne . Acetylene is the common term for ethyne.
Structure of ethyne, C2H2
- The naming of C3H4 The letter prop denotes the presence of three carbon atoms in this molecule. This hydrocarbon is unsaturated because it has a carbon-carbon triple bond. The yne at the ending is used to denote a triple bond. When the letters prop and yne are combined, the IUPAC nomenclature for this combination is propyne . Propyne has a common name known as methyl-acetylene.
Structure of Propyne, C3H4
What Is A Hydrocarbon
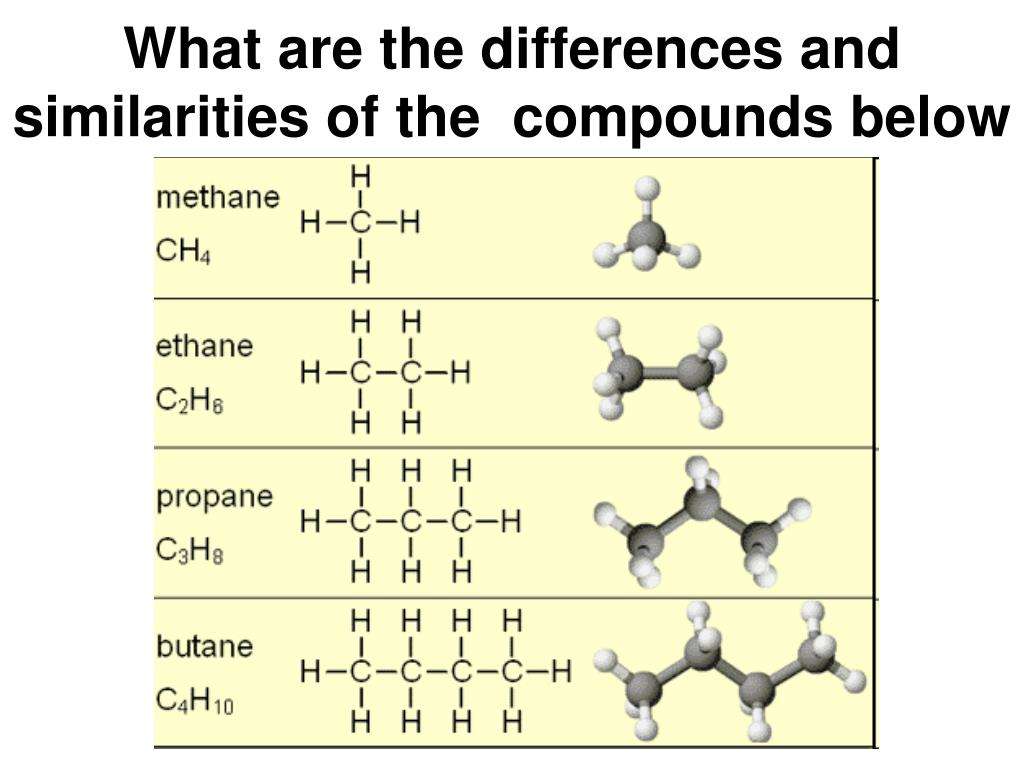
A hydrocarbon is an organic chemical compound composed exclusively of hydrogen and carbon atoms. Hydrocarbons are naturally-occurring compounds and form the basis of crude oil, natural gas, coal, and other important energy sources.
Hydrocarbons are highly combustible and produce carbon dioxide, water, and heat when they are burned. Therefore, hydrocarbons are highly effective as a source of fuel.
You May Like: Beth Thomas Documentary
Naming Of Unsaturated Hydrocarbons With Double Bond
- The naming of C2H4 The letter eth denotes the existence of two carbon atoms in this molecule. This hydrocarbon is unsaturated because it has a carbon-carbon double bond. The ene at the ending is used to denote a double bond. When the letters eth and ene are combined, the IUPAC name for this combination is ethene . Ethene has a common name known as ethylene.
Structure of ethene C2H4
- The naming of C3H6 The letter prop denotes the presence of three carbon atoms in this molecule. This hydrocarbon is unsaturated because it has a carbon-carbon double bond. The ene at the ending is used to denote a double bond. When the letters prop and ene are combined, the IUPAC nomenclature for this combination is propene . Propane has a common name known as propylene.
Structure of Propene, C3H6
Are Hydrocarbons Harmful To Humans
Yes, hydrocarbons are dangerous to humans. Gases emitted from hydrocarbons have shown to damage respiratory systems and harm the environment through climate change and the greenhouse effect. Oil spills damage ecosystems. While hydrocarbons are natural occurrences, it is their manipulation into energy sources that are harmful to humans.
Don’t Miss: Geometry Dash Hack Steam
Naming Of Saturated Hydrocarbons
- The naming of CH4 This chemical has one carbon atom, which is denoted by meth. Its saturated since its made up entirely of single bonds. The ane at the end denotes a saturated hydrocarbon. When the meth and ane are combined, the IUPAC nomenclature for this substance is methane . Methane is the IUPAC name as well as the common name for the hydrocarbon CH4.
Structure of Methane CH4
- The naming of C2H6 The letter eth denotes the presence of two carbon atoms in this molecule. Its saturated since its made up entirely of single bonds. The ane at the end denotes a saturated hydrocarbon. When eth and ane are combined, the IUPAC name for this combination is ethane . The IUPAC and common names for the hydrocarbon C2H6 are identical, ethane.
Structure of Ethane C2H6
Examples Of Hydrocarbon In A Sentence
hydrocarbon Wiredhydrocarbon The Salt Lake Tribunehydrocarbon clevelandhydrocarbon CNNhydrocarbon The New Yorkerhydrocarbon WSJhydrocarbon Washington Posthydrocarbon WSJ
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘hydrocarbon.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Recommended Reading: Difference Between Molecular And Electronic Geometry
Examples Of Hydrocarbon Companies
Because hydrocarbons are the largest energy source in the globe, some of the largest companies in the world are hydrocarbon companies. These primarily include oil and gas companies that mine hydrocarbons and convert them into the energy sources that the world uses to power almost everything.
Some of the largest hydrocarbon companies include Exxon Mobil, Chevron, Royal Dutch Shell, Saudi Aramco, and PetroChina. The success of these companies and their ability to provide energy sources efficiently and cheaply greatly impact the world’s financial markets and economies.
The fluctuations in the price of oil have a great impact on the cost of gasoline for cars, jet fuel for airlines, and gas for heating homes. These costs affect how consumers spend their money decisions that reverberate throughout the global economy.
How Reactive Are Hydrocarbons
Hydrocarbons are well known for inertness, especially the saturated members of hydrocarbons are inert. However, others will undergo three major types of reactions mentioned as follows:
Substitution Reaction: This kind of reaction is undergone by aromatic compounds. For example: Benzene and ethylene react to form ethylbenzene.
Addition Reaction: This kind of reaction is undergone by alkenes and alkynes and a variety of reagents add across the pi-bonds. Such reagents include hydrogen chloride, chlorine and hydrogen. Alkenes and alkynes undergo alkyne metathesis, polymerization and alkene metathesis.
Combustion: When hydrocarbons are combusted, the energy produced are the heat sources and electrical energy. It can be used as home heaters that make use of petroleum or natural gas. In the presence of oxygen, hydrocarbons produce steam, carbon dioxide and heat during combustion.
Read Also: Hawkes Learning Systems Answer Key College Algebra
What Are Hydrocarbons
Simplest organic compounds, hydrocarbons are majorly studied in organic chemistry and it is an organic compound that bears only hydrogen and carbon atoms. Those hydrocarbons from where one hydrogen atom is removed are called hydrocarbons and these represent the functional groups. Hydrocarbons are usually colourless, hydrophobic and have only weak odours.
We can find hydrocarbons in nature and besides fossil fuels, these are present in trees and plants. For example, in carrots and green leaves, these are available in the form of carotenoids that are known as pigments. Fuel in buses and autos, CNG, cooking gas and LPG all consist of hydrocarbons. A hydrocarbon polymer or hydrocarbon chain is a chain-like molecule that consists of multiple units which are linked together and an example of it is such that over 98 percent of natural crude rubber. The chemistry and structure of individual hydrocarbons is dependent on the types of chemical bonds linking the atoms of their constituent molecules.
What Are Isomers
A molecular formula represents only one substance in inorganic chemistry. For example, HSO stands for sulphuric acid, which is a single compound. However, in organic chemistry, a single molecular formula can be used to represent two or more distinct molecules. This is because the identical carbon atoms in organic molecules can be arranged in a variety of ways to produce distinct structures and thus different compounds. In organic chemistry, for example, the same chemical formula C4H10 can represent two different compounds: normal-butane and iso-butane. The following example will help to clarify this topic.
- Consider the chemical molecule butane . This chemical has four carbon atoms that can be linked in two ways to create two different structures. To begin, all four carbon atoms are connected in a continuous straight chain to form the structure seen below. The compound normal butane, abbreviated as n-butane, is represented by this structure.
- In the second case, three carbon atoms can be combined in a straight chain and the fourth carbon atom can be joined in a side chain, resulting in the structure illustrated below. Iso-butane is the chemical that has this structure.
Don’t Miss: Kim Kardashian’s Biological Father